Abstract
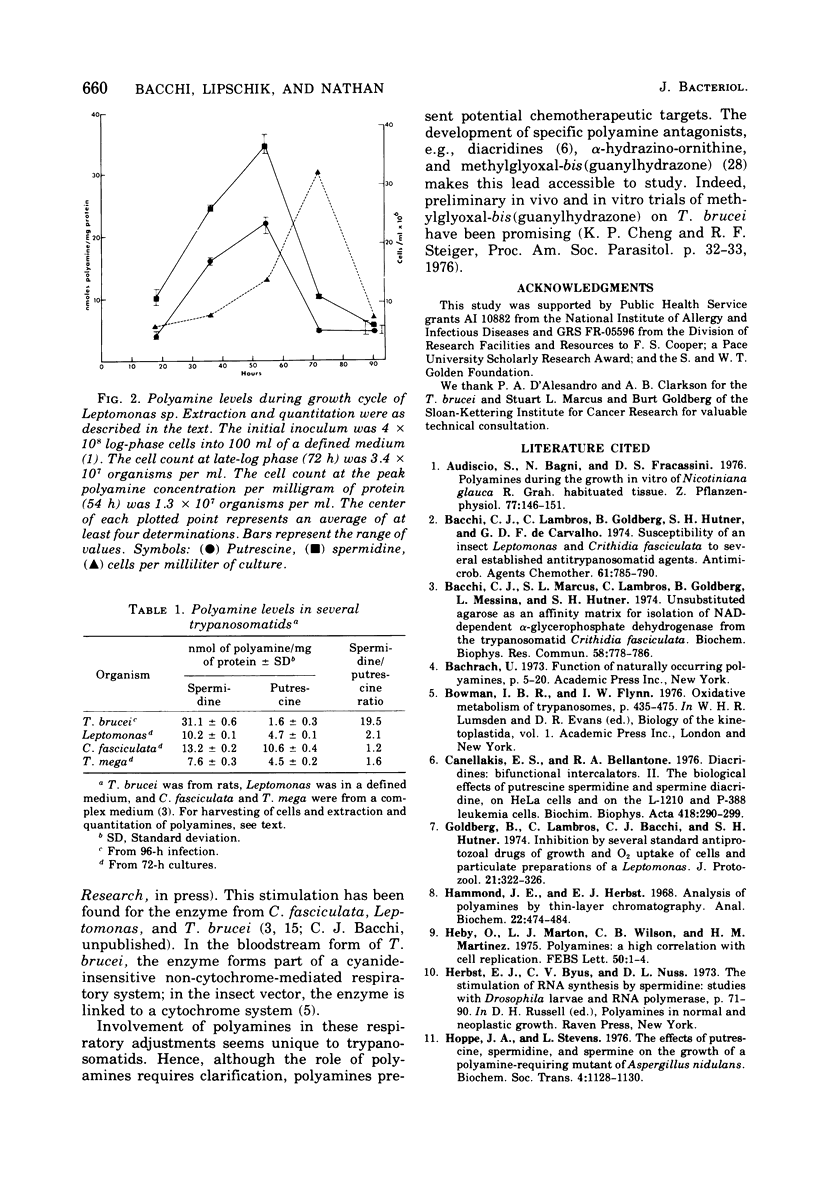
Polyamines were determined by n-butanol extraction and thin-layer chromatography in four trypanosomatids: Trypanosoma brucei (rat infection) and cultures of Crithidia fasciculata, Leptomonas sp., and Trypanosoma mega. All had putrescine and spermidine but no detectable spermine. Putrescine and spermidine levels were quantitated for extracts of leptomonas during the normal growth cycle. Spermidine values peaked 18 h before peak cell populations. Spermidine-putrescine ratios for all organisms were related to the presumed phylogeny of the group.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchi C. J., Marcus S. L., Lambros C., Goldberg B., Messina L., Hutner S. H. Unsubstituted agarose as an affinity matrix for isolation of NAD-dependent alpha-glycerophosphate dehydrogenase from the trypanosomatid Crithidia fasciculata. Biochem Biophys Res Commun. 1974 Jun 4;58(3):778–786. doi: 10.1016/s0006-291x(74)80485-7. [DOI] [PubMed] [Google Scholar]
- Bacchi J., Lambros C., Goldberg B., Hutner S. H., de Carvalho G. D. Susceptibility of an insect Leptomonas and Crithidia fasciculata to several established antitrypanosomatid agents. Antimicrob Agents Chemother. 1974 Dec;6(6):785–790. doi: 10.1128/aac.6.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canellakis E. S., Bellantone R. A. Diacridines: bifunctional intercalators. II. The biological effects of putrescine, sperimidine and spermine diacridines on HeLa cells and on the L-1210 and P-388 leukemia cells. Biochim Biophys Acta. 1976 Feb 5;418(3):290–299. doi: 10.1016/0005-2787(76)90291-4. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Lambros C., Bacchi C. J., Hutner S. H. Inhibition by several standard antiprotozoal drugs of growth and O2 uptake of cells and particulate preparations of a Leptomonas. J Protozool. 1974 May;21(2):322–326. doi: 10.1111/j.1550-7408.1974.tb03662.x. [DOI] [PubMed] [Google Scholar]
- Hammond J. E., Herbst E. J. Analysis of polyamines by thin-layer chromatography. Anal Biochem. 1968 Mar;22(3):474–484. doi: 10.1016/0003-2697(68)90288-1. [DOI] [PubMed] [Google Scholar]
- Heby O., Marton L. J., Wilson C. B., Martinez H. M. Polyamines: a high correlation with cell replication. FEBS Lett. 1975 Jan 15;50(1):1–4. doi: 10.1016/0014-5793(75)81026-x. [DOI] [PubMed] [Google Scholar]
- Hope J. A., Stevens L. The effects of putrescine, spermidine, and spermine on the growth of a polyamine-requiring mutant of Aspergillus nidulans. Biochem Soc Trans. 1976;4(6):1128–1130. doi: 10.1042/bst0041128. [DOI] [PubMed] [Google Scholar]
- Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977 Jan 11;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Hara K., Watanabe Y., Hirose S., Takeda Y. Polyamine and magnesium contents and polypeptide synthesis as a function of cell growth. Biochem Biophys Res Commun. 1975 Jan 2;64(3):897–904. doi: 10.1016/0006-291x(75)90132-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambros C., Bacchi C. J., Marcus S. L., Hutner S. H. Paradoxical activation of Leptomonas NAD-linked alpha-glycerophosphate dehydrogenase by ethidium and antrycide. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1227–1234. doi: 10.1016/0006-291x(77)91649-7. [DOI] [PubMed] [Google Scholar]
- Lambros C., Bacchi C. J. Properties of a purified NAD-linked alpha-glycerophosphate dehydrogenase from Leptomonas sp.; activation by polyamines. Biochem Biophys Res Commun. 1976 Feb 9;68(3):658–667. doi: 10.1016/0006-291x(76)91196-7. [DOI] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Rusch H. P. Regulation of polyamine synthesis in Physarum polyciphalum during growth and differentiation. Biochim Biophys Acta. 1973 Feb 28;297(2):503–516. doi: 10.1016/0304-4165(73)90098-6. [DOI] [PubMed] [Google Scholar]
- Nickerson K. W., Dunkle L. D., Van Etten J. L. Absence of spermine in filamentous fungi. J Bacteriol. 1977 Jan;129(1):173–176. doi: 10.1128/jb.129.1.173-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Eloranta T., Kajander O. Biosynthesis and metabolism of polyamines and S-adenosylmethionine in the rat. Biochem Soc Trans. 1976;4(6):968–971. doi: 10.1042/bst0040968. [DOI] [PubMed] [Google Scholar]
- Raina A., Jansen M., Cohen S. S. Polyamines and the accumulation of ribonucleic acid in some polyauxotrophic strains of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1684–1696. doi: 10.1128/jb.94.5.1684-1696.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolle I., Hobucher H-E, Kneifel H., Paschold B., Riepe W., Soeder C. J. Amines in unicellular green algae. 2. Amines in Scenedesmus acutus. Anal Biochem. 1977 Jan;77(1):103–109. doi: 10.1016/0003-2697(77)90294-9. [DOI] [PubMed] [Google Scholar]
- Smith T. A. The oxidation of polyamines in higher plants. Xenobiotica. 1971 Jul-Oct;1(4):449–450. doi: 10.3109/00498257109041508. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Biosynthesis and metabolism of 1,4-diaminobutane, spermidine, spermine, and related amines. Adv Enzymol Relat Areas Mol Biol. 1972;36:203–268. doi: 10.1002/9780470122815.ch7. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Corti A., Tadolini B. On the development of specific inhibitors of animal polyamine biosynthetic enzymes. Ital J Biochem. 1976 Jan-Feb;25(1):5–32. [PubMed] [Google Scholar]