Abstract
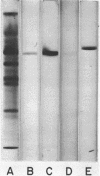
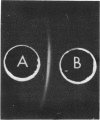
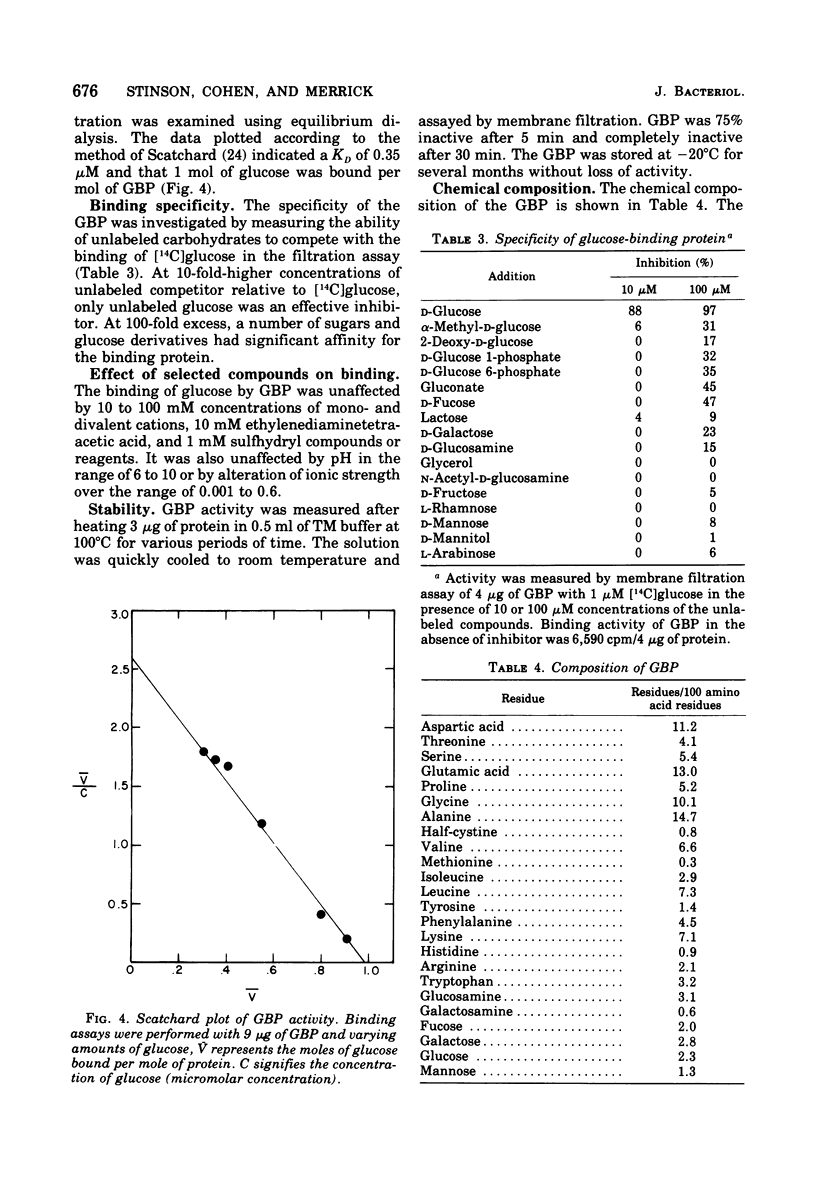
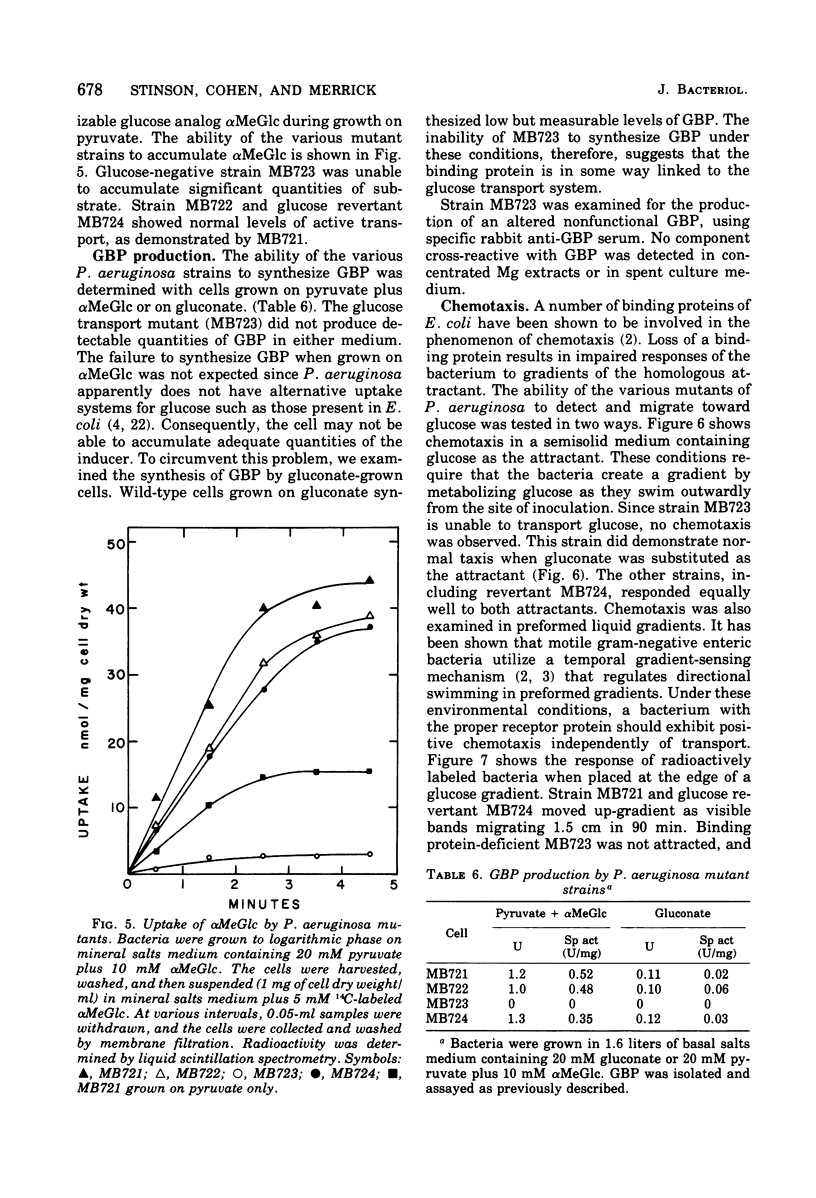
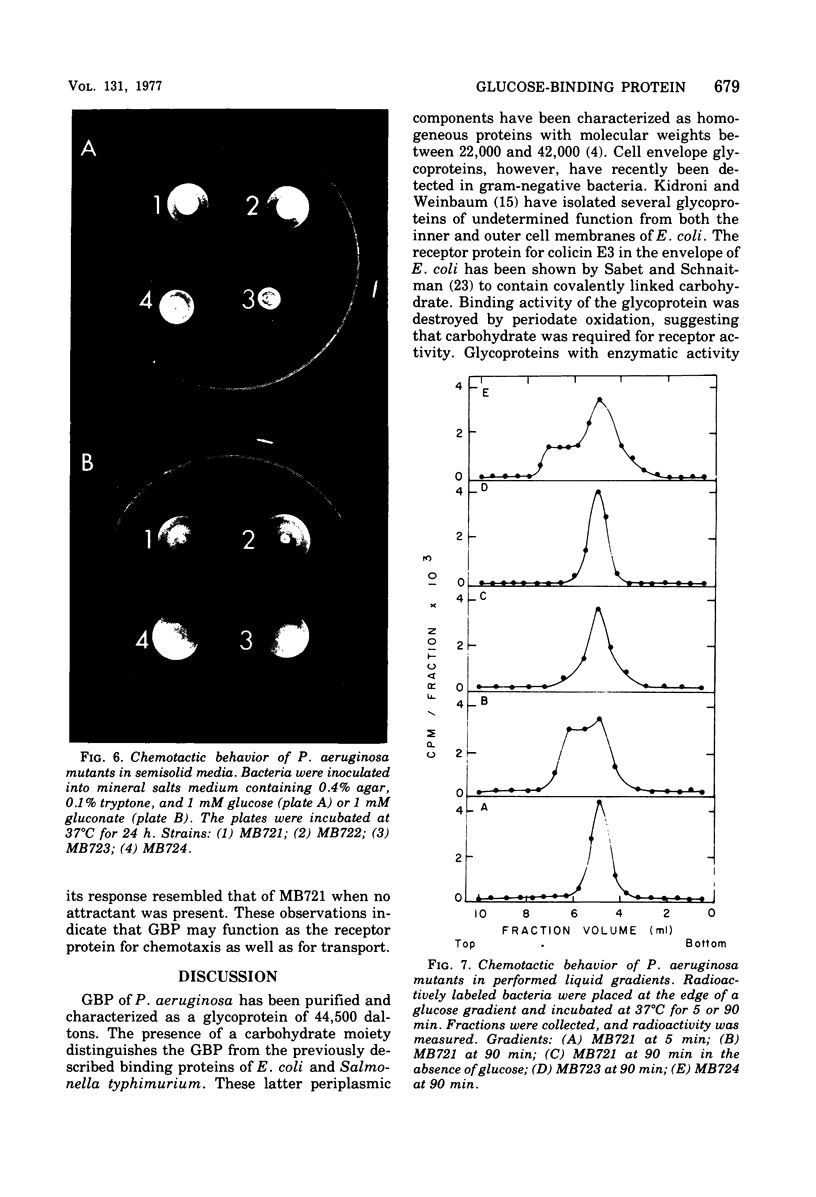
A glucose-binding glycoprotein (GBP) from the periplasm of Pseudomonas aeruginosa was purified to homogeneity as judged by polyacrylamide gel electrophoresis, molecular sieve chromatography, and double-diffusion gel precipitation. It had an average molecular weight of 44,500 and an isoelectric point of 4.7. One mole of glucose was bound per mole of GBP with a dissociation constant of 0.35 μM. The binding of radioactive glucose by GBP was not significantly inhibited by 10-fold-higher concentrations of other carbohydrates; however, a number of related compounds were found to compete at 100-fold-higher concentrations. Amino acid analyses revealed predominant amounts of alanine, glutamate, and glycine and a low content of sulfur-containing amino acids. The carbohydrate moiety of GBP, comprising nearly 16% of the total weight, contained galactosamine, glucosamine, fucose, galactose, glucose, and mannose. A GBP-deficient mutant, strain MB723, was found to be defective in both membrane transport and glucose chemotaxis. Strain MB724, a revertant to GBP-positive phenotype, simultaneously recovered normal levels of both membrane functions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Aswad D., Koshland D. E., Jr Isolation, characterization and complementation of Salmonella typhimurium chemotaxis mutants. J Mol Biol. 1975 Sep 15;97(2):225–235. doi: 10.1016/s0022-2836(75)80036-2. [DOI] [PubMed] [Google Scholar]
- Boos W. Bacterial transport. Annu Rev Biochem. 1974;43(0):123–146. doi: 10.1146/annurev.bi.43.070174.001011. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eagon R. G., Phibbs P. V., Jr Kinetics of transport of glucose, fructose, and mannitol by Pseudomonas aeruginosa. Can J Biochem. 1971 Sep;49(9):1031–1041. doi: 10.1139/o71-151. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Eagon R. G. Transport of glucose, gluconate, and methyl alpha-D-glucoside by Pseudomonas aeruginosa. J Bacteriol. 1974 Mar;117(3):1261–1269. doi: 10.1128/jb.117.3.1261-1269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Independent regulation of hexose catabolizing enzymes and glucose transport activity in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1041–1048. doi: 10.1016/0006-291x(72)90813-3. [DOI] [PubMed] [Google Scholar]
- KORMAN R. Z., BERMAN D. T. Medium for the differentiation of acid producing colonies of staphylococci. J Bacteriol. 1958 Oct;76(4):454–455. doi: 10.1128/jb.76.4.454-455.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Carbohydrate structure of glycopeptides isolated from an hepatic membrane-binding protein specific for asialoglycoproteins. J Biol Chem. 1976 Sep 10;251(17):5292–5299. [PubMed] [Google Scholar]
- Kidroni G., Weinbaum G. Glucosamine-labelled envelope proteins of Escherichia coli K-12. II. Location in inner and outer membranes. Biochim Biophys Acta. 1975 Aug 13;399(2):447–459. doi: 10.1016/0304-4165(75)90272-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Procedures for the chromatographic determination of amino acids on four per cent cross-linked sulfonated polystyrene resins. J Biol Chem. 1954 Dec;211(2):893–906. [PubMed] [Google Scholar]
- Midgley M., Dawes E. A. The regulation of transport of glucose and methyl alpha-glucoside in Pseudomonas aeruginosa. Biochem J. 1973 Feb;132(2):141–154. doi: 10.1042/bj1320141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Properties of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):517–526. doi: 10.1128/jb.117.2.517-526.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L. Membrane transport. Annu Rev Biochem. 1972;41(10):777–814. doi: 10.1146/annurev.bi.41.070172.004021. [DOI] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Purification and properties of the colicin E3 receptor of Escherichia coli. J Biol Chem. 1973 Mar 10;248(5):1797–1806. [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Cohen M. A., Merrick J. M. Isolation of dicarboxylic acid- and glucose-binding proteins from Pseudomonas aeruginosa. J Bacteriol. 1976 Nov;128(2):573–579. doi: 10.1128/jb.128.2.573-579.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart W. D., Debusk A. G. Molecular transport. I. In-vitro studies of isolated glycoprotein subunits of the amino acid transport system of Neurospora crassa conidia. Arch Biochem Biophys. 1971 Jun;144(2):512–518. doi: 10.1016/0003-9861(71)90356-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wrigley C. W. Analytical fractionation of plant and animal proteins by gel electrofocusing. J Chromatogr. 1968 Aug 27;36(3):362–365. doi: 10.1016/s0021-9673(01)92959-0. [DOI] [PubMed] [Google Scholar]
- Yamane K., Suzuki H., Nisizawa K. Purification and properties of extracellular and cell-bound cellulase components of Pseudomonas fluorescens var. cellulosa. J Biochem. 1970 Jan;67(1):19–35. doi: 10.1093/oxfordjournals.jbchem.a129231. [DOI] [PubMed] [Google Scholar]