Abstract
To investigate the functional role of different α1-adrenergic receptor (α1-AR) subtypes in vivo, we have applied a gene targeting approach to create a mouse model lacking the α1b-AR (α1b−/−). Reverse transcription–PCR and ligand binding studies were combined to elucidate the expression of the α1-AR subtypes in various tissues of α1b +/+ and −/− mice. Total α1-AR sites were decreased by 98% in liver, 74% in heart, and 42% in cerebral cortex of the α1b −/− as compared with +/+ mice. Because of the large decrease of α1-AR in the heart and the loss of the α1b-AR mRNA in the aorta of the α1b−/− mice, the in vivo blood pressure and in vitro aorta contractile responses to α1-agonists were investigated in α1b +/+ and −/− mice. Our findings provide strong evidence that the α1b-AR is a mediator of the blood pressure and the aorta contractile responses induced by α1 agonists. This was demonstrated by the finding that the mean arterial blood pressure response to phenylephrine was decreased by 45% in α1b −/− as compared with +/+ mice. In addition, phenylephrine-induced contractions of aortic rings also were decreased by 25% in α1b−/− mice. The α1b-AR knockout mouse model provides a potentially useful tool to elucidate the functional specificity of different α1-AR subtypes, to better understand the effects of adrenergic drugs, and to investigate the multiple mechanisms involved in the control of blood pressure.
The adrenergic receptors (ARs) mediate the physiological effects of the catecholamines epinephrine and norepinephrine by coupling to several of the signaling pathways modulated by G proteins. The AR family includes nine different gene products, three β (β1, β2, β3), three α2 (α2-C10, α2-C4, α2-C2), and three α1 (α1a, α1b, α1d) receptor subtypes. The ARs share similar structural features characterized by the seven-transmembrane domain motif common to other G protein-coupled receptors.
A variety of physiological effects of catecholamines are mediated by the α1-AR subtypes, including the control of blood pressure, glycogenolysis, and the contractility of the urinary tract (1). Heterogeneity of the α1-AR initially was suggested by various pharmacological studies and confirmed by molecular cloning of three α1-AR subtypes, as reviewed in ref. 2. The alignment of the cloned and pharmacologically defined α1-AR subtypes has been the object of some controversy recently solved with the contribution of several studies (3, 4).
After the discovery of α1-AR heterogeneity, a variety of studies have attempted to assess whether the different α1-AR-mediated responses in various organs could be assigned to distinct subtypes that might differ in their signaling and/or regulatory properties. To address this question, the tissue distribution of the mRNA encoding the three α1-AR subtypes has been investigated in various species, including humans and rat (3, 4), using Northern blot analysis, reverse transcription–PCR (RT-PCR), or RNase protection assay. The mRNA of different α1-AR subtypes has been found in several organs, including brain, heart, liver, kidney, spleen, blood vessels, vas deferens, and prostate. However, the level of expressed mRNA does not necessarily reflect the expression of the receptor protein.
In vivo studies aiming to assess a specificity of the functional responses mediated by distinct α1-AR subtypes have been hampered by the fact that the subtype-selective drugs are only moderately selective and might interact with other adrenergic as well as nonadrenergic receptors. Thus, the functional implications of α1-AR heterogeneity and their physiological relevance remain largely unknown.
To contribute to the elucidation of the physiological role of the α1-AR subtypes in vivo we have used gene targeting to create a mouse model lacking the α1b-AR. Recently, targeted gene disruption has been increasingly used to elucidate the in vivo functions of several receptors, including some AR subtypes (5–8). The potential functional changes occurring in the knockout mice might allow, on one hand, to assign distinct functions to the receptor that has been deleted, and on the other, to test the functional redundancy among receptor subtypes.
In this study, we describe the cloning and gene targeting of the mouse α1b-AR as well as the initial functional characterization of the knockout mice lacking this receptor subtype. Our findings identify the α1b-AR as a mediator of the vascular contractile and blood pressure responses.
MATERIALS AND METHODS
Cloning of the Mouse α1B-AR gene and cDNA.
After the screening of a 129/Sv mouse genomic library (Stratagene) using the hamster α1b-AR cDNA as a probe, one positive clone was obtained. The HindIII restriction fragment of the genomic clone was subcloned in pBlueScript II SK and sequenced with primers derived from the hamster α1b-AR cDNA. The genomic clone contained the first exon of the mouse α1b-AR encoding amino acids 1–316 of the receptor. To obtain the full-length mouse α1b-AR cDNA, a BALB/c mouse brain cDNA library was screened with the hamster α1b-AR cDNA as a probe. The single cDNA clone obtained was missing the N terminus. Thus, the expression vector containing the full-length mouse cDNA was constructed ligating the pRK5 with amino terminal and carboxyl terminal restriction fragments derived from the genomic and cDNA clones, respectively (further information is available upon request). Library screening and DNA sequencing were as described (9).
Gene Targeting.
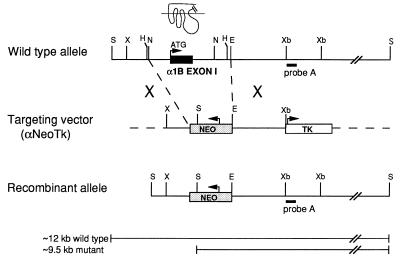
A ≈7.5-kb restriction fragment of the mouse α1b-AR genomic clone (Fig. 1) was subcloned into pBlueScript. The ≈2.6-kb NcoI–EcoRV restriction fragment including the first exon of the α1b-AR was replaced with a 1.6-kb cassette containing the neomycin resistance gene (neo) under the control of the phosphoglycerate kinase promoter, as described in ref. 10. In addition, the 1.8-kb herpes simplex virus thymidine kinase poly(A) cassette was inserted at the XbaI site to obtain the targeting vector α1NeoTk (Fig. 1). After its linearization with XmnI, the targeting vector contained two regions of homology with the α1b-AR gene: ≈0.9 kb and ≈2.1 kb of the 5′ and 3′ untranslated sequences flanking the first exon, respectively. The linearized targeting vector was electroporated into 129 (HM-1) embryonic stem cells (ES), described in ref. 10, which then were subjected to double selection with G418 and gancyclovir. Southern blot analysis was performed on 140 resistant ES clones, two of which were positive for the targeting event. Genomic DNA was digested with SacI, electrophoresed on 0.8% agarose gel, transferred, and hybridized with the 0.3-kb probe A derived from the α1b-AR locus (Fig. 1). Digestion of the genomic DNA with SacI generated ≈12-kb and ≈9.5-kb restriction fragments for the wild-type and disrupted allele, respectively. One of the two positive ES clones was expanded and microinjected into C57BL/6J mouse blastocysts (10), which then were transferred into pseudopregnant NMRI females. Two of seven chimeric mice that were mated gave rise to germ-line transmission of the disrupted allele. Male and females with different genotypes and from different litters were randomly intercrossed to obtain α1b +/+, +/−, and −/− progeny. The mouse tail genomic DNA was screened by Southern analysis, as described. The studies described below were performed on mice belonging to generations F3 to F5.
Figure 1.
Targeted disruption of the mouse α1b-AR gene. The structure of the wild-type α1b-AR allele, the targeting vector (αNeoTk), and the recombinant allele after homologous recombination are shown. Exon I (filled box) encodes the receptor portion from its starting methionine to transmembrane domain VI, as indicated by the schematic receptor structure. The neo cassette (gray box) replacing exon I introduces an additional SacI restriction site, which was used for Southern analysis. Probe A (black bar) was used for Southern blot analysis after digestion of DNA with SacI. The solid bars indicate the expected fragment sizes of the wild-type and mutant α1b-AR alleles. E, EcoRI; H, HindIII; N, NcoI; S, SacI; X, XmnI; Xb, XbaI.
RT-PCR Analysis.
Total RNA from different mouse tissues as well as from COS-7 cells expressing the hamster α1b, rat α1d, and bovine α1a-AR was prepared using the RNeasy Plant Total RNA kit (Qiagen). Two micrograms of total RNA were reverse-transcribed for 60 min at 37°C in a 30-μl reaction mixture containing 50 mM Tris⋅HCl at pH 8.3, 75 mM KCl, 3 mM MgCl2, 100 pmol oligo(dT)12–18 (GIBCO), 300 pmol of random hexamers (Pharmacia), 10 mM DTT, 40 units of RNasin (Promega), 0.5 mM of each dNTP, and 400 units of Superscript reverse-transcriptase (GIBCO/BRL). One-tenth of each cDNA sample was amplified by PCR with receptor-specific primers set together with a primer set specific for the hypoxanthine-phosphoribosyl-transferase (11). Each sample contained the upstream and downstream primers (20 pmol of each), 0.25 mM of each dNTP, 50 mM KCl, 10 mM Tris⋅HCl at pH 8.6, 1.5 mM MgCl2, and 1.25 units of Taq DNA polymerase (Boehringer). PCR amplification of the α1d and α1b cDNA was performed by adding 10% dimethyl sulfoxide in the reaction mix. Thermal cycling was performed for 2 min at 94°C, 1 min at 56°C, and 2 min at 72°C for 40 cycles. The receptor-specific primers were derived from regions upstream (corresponding to the third intracellular loop of the receptor) and downstream of the intron (corresponding to the C-tail of the receptor) to avoid the amplification of genomic DNA. The upstream and downstream primers (5′-3′ direction) were AGGTGGTTCTGAGGATCCACTGTC and CGGAACTTATGGGACAGGCTGGA for the α1d, CCACTCTAAGAACTTTCATGAGGACACC and ATGCAGCTGCCACTGTCATCCAGAGAGT for the α1b, and CCAGCGCCAAGAACAAGACGCACTTCTC and TCATTCACAGACCCCATCCGTCTTGGAGAT for the α1a, respectively. The primers were derived from the mouse α1d (12), mouse α1b, and bovine α1a-AR (13). The hypoxanthine-phosphoribosyl-transferase primers (5′-3′ direction) were GATTATGGACAGGACTGAAAGAC upstream and CGAGAGGTCCTTTTCACCAGCAAG downstream. Control PCR reactions also were performed on nontranscribed RNA to exclude any contamination by DNA. The specificity of the amplified DNA fragments was determined by Southern blot using receptor-specific 32P-labeled probes [the 1.5-kb StuI–PstI fragment of the rat α1d-AR (14), the 0.3-kb BamHI–BssHII fragment of the hamster α1b-AR (9), and the 0.9-kb NcoI–PvuII fragment of the bovine α1a-AR (13)].
Ligand Binding.
Mouse tissues were minced in 10 ml of ice-cold buffer (5 mM Tris/5 mM EDTA, pH 7.4) and homogenized with an Ultra-Turrax (Janke & Kunkel, Stauffen, Germany). The homogenates were filtered through four layers of medical gauze and centrifuged for 20 min at 10,000× g at 4°C. The pellets were washed once by centrifugation and resuspended in binding buffer (50 mM Tris/0.5 mM EDTA, pH 7.4). Radioligand binding was measured using [3H]prazosin, [3H]RX821002, and [125I]cyano pindolol (DuPont-NEN) as described (8, 15). Binding studies and measurement of inositol phosphates in COS-7 cells expressing the mouse α1b-AR were performed as described (9). All curve-fitting procedures were performed using the inplot program (GraphPAD, San Diego).
Blood Pressure Measurement.
After cervical incision on mice anesthetised with halothane (1–2% in oxygen), two catheters (PE-10 tubing) were inserted, one into the right carotid artery for blood pressure measurement and the other into the jugular vein for drug injection (16). The vessels then were ligated, and the catheters were tunnelled subcutaneously to exit at the back of the neck. The skin incision was closed, and mice were allowed to recover for 3 hr. After placing them in Plexiglas tubes for 30 min to partially restrict their movements, the arterial line was connected to a pressure transducer, and mean arterial blood pressure was recorded with a computerized data-acquisition system as described (17). Phenylephrine (Sigma) and norepinephrine (Sigma) were dissolved in saline, and increasing doses were administered in a volume of 100 μl at 15–20 min intervals to allow blood pressure and heart rate to return to baseline values. Angiotensin II was from CIBA–Geigy and vasopressin from Sandoz Pharmaceutical. Antagonists REC2739 and 3016 (Recordati, Milan, Italy) were dissolved in 10% polyethylene glycol 400 and administered in a volume of 100 μl.
Aorta Contractility.
Aortic rings were prepared as described (18). On the day of the experiment, the mice were weighed and decapitated. The thoracic aorta was excised and placed in cold Krebs–Henseleit bicarbonate buffer (118.3 mM NaCl/4.7 mM KCl/2.5 mM CaCl2/1.2 mM MgSO4/1.2 mM KH2PO4/25 mM NaHCO3/5.6 mM glucose). The aorta was cleaned of adhering perivascular tissue and cut into 2-mm long rings. These rings were suspended in isolated tissue baths filled with 20 ml of the above buffer continuously bubbled with a mixture of 5% CO2-95%O2 (pH 7.37–7.42) at 37°C. One end of the aortic ring was connected to a tissue holder and the other to an isometric force transducer. The signal was transmitted to a Gould (Cleveland) pressure processor and then into a computerized system by Gould’s data acquisition and signal analysis. The analysis of the generated curves was performed by the view II software (Gould), and the sensitivity of the system was 5 ± 1 mg of tension generated. Rings were equilibrated for 90 min in the unstretched condition, and the buffer replaced every 20 min. The length of the smooth muscle was increased stepwise during the equilibration period to adjust passive wall tension to 0.5 g. This tension was found to be optimal in both the α1b +/+ and −/− mice for aorta contraction of 28–35 g mice induced by serotonin (10−6 M). Once basal tension was established, the length of the rings was not modified. Caution was made to avoid endothelial damage whose functional integrity was assessed using 10−7 M acetylcholine (results not shown).
RESULTS
Cloning of the Mouse α1B-AR gene and cDNA.
To construct a targeting vector to disrupt the mouse α1b-AR gene we screened a 129/Sv mouse genomic library and isolated a single genomic clone containing the first exon of the mouse α1b-AR. The restriction and sequencing analysis of this clone has revealed that the genomic structure of the mouse α1b-AR gene is similar to that reported for the homologous gene in hamster (9) and humans (19), the bovine α1a-AR (13), and all three human α1-AR subtypes (20). Most of the receptor protein from its starting methionine to transmembrane domain VI is coded by a single exon interrupted by one large intron located in the middle of transmembrane domain VI. The 5′-flanking sequence (750 bp) (sequence deposited in the EMBL database with accession no. Y12738) of the first exon containing the putative promoter and transcription initiation sites displays 85% identity with the homologous region of the human gene (19).
To characterize the pharmacological properties of the mouse α1b-AR, we cloned its cDNA and expressed it in COS-7 cells. The mouse α1b-AR cDNA (sequence deposited in the EMBL database with accession no. Y12738) displays 99.3, 99.3, and 98.2% identity with its hamster (9), rat (21), and human (19) homologues, respectively. Both the maximal receptor expression and the binding affinities of prazosin, 5-methylurapidil, phenylephrine, and norepinephrine of the mouse α1b-AR expressed in COS-7 cells were similar to those previously reported for its hamster homologue (results not shown). Stimulation of COS-7 cells expressing the mouse α1b-AR with epinephrine resulted in 450% increase of inositol phosphate levels above basal as previously reported for the hamster receptor (9).
Targeted Disruption of the Mouse α1b-AR gene.
The strategy for inactivating one copy of the α1b-AR gene in ES cells is described in Fig. 1. Homologous recombinants were identified by Southern analysis of genomic DNA and microinjected into C57BL/6J blastocyst stage embryos. Two of seven chimeric mice that were mated gave rise to germ-line transmission of the disrupted allele. Analysis of the α1b-AR genotype frequencies after intercrosses of heterozygous mutant mice did not reveal any deviation from the Mendelian expectations (results not shown). Monitoring of the mice body weight from days 2–3 to 20 weeks of postnatal life did not reveal any significant difference in growth among mice of different α1b genotypes. Thus, the disruption of the α1b-AR gene does not seem to have any major effect on mouse development, fertility, growth, or feeding behavior under standard breeding conditions.
mRNA Expression of the α1-AR Subtypes in α1b +/+ and −/− mice.
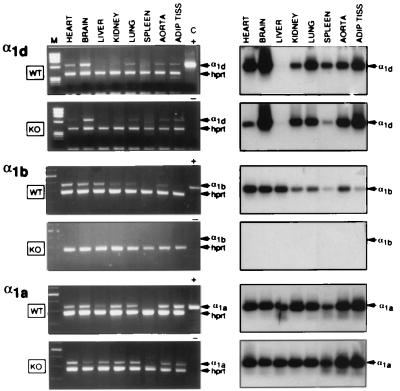
Because of the low abundancy of the mRNA levels for different α1-AR subtypes in various animal species (4), RT-PCR was used to assess the mRNA expression of the α1d, α1b, and α1a-AR in various tissues of male α1b +/+ and −/− mice. As shown in Fig. 2, in α1b+/+ mice the α1b-AR is expressed in all tissues investigated with apparently lower abundancy in spleen and adipose tissue, whereas the expression of the α1a-AR seems uniform. The α1d-AR also is expressed in all tissues of the α1b+/+ mice, except in liver. Because our RT-PCR analysis is semiquantitative, the intensity of the signals corresponding to the amplified products can provide only an approximate measure of the abundancy of each mRNA transcript. However, the complete lack of amplification of the α1b-AR mRNA in α1b−/− mice confirms that the knockout of the α1b-AR gene was successful. The expression of the α1d and α1a-AR in the α1b−/− mice appeared similar to that in α1b+/+ (Fig. 2), suggesting that the inactivation of the α1b-AR gene does not have any dramatic compensatory effect on the expression of the other α1-AR subtypes.
Figure 2.
RT-PCR analysis of the RNA from different tissues of α1b +/+ (WT, wild type) and −/− (KO, knockout) mice. (Left) Ethidium bromide staining of the RT-PCR fragments. The α1d, α1b, and α1a mRNA transcripts were detected as 650-, 470-, and 450-bp fragments, respectively. RT-PCR analysis was controlled by detection of the 390-bp fragment of the hypoxanthine-phosphoribosyl-transferase message. The DNA size markers (M) are shown on the left. The positive control (C +) indicates the RT-PCR analysis of RNA derived from COS-7 cells expressing each α1-AR subtype. For the negative control (C −), RT-PCR analysis was performed on samples without RNA. (Right) Southern blots of the RT-PCR fragments shown on the left. The specificity of the amplified fragments was assessed using 32P-labeled probes specific for each receptor subtype (see Materials and Methods).
Adrenergic Pharmacology in αib +/+ and −/− mice.
Saturation binding analysis showed that the Kd value of the α1-antagonist [3H]prazosin was ≈100 pM in all tissues explored for both α1b +/+ and −/− mice (results not shown). On the other hand, receptor density (Bmax) was significantly reduced in several tissues of the α1b−/− mice, except in kidney (Table 1). The loss of α1-AR in α1b−/− as compared with α1b+/+ mice was 98% in liver, 74% in heart, 42% in cerebral cortex, and 32% in cerebellum. These findings indicate that the inactivation of the α1b-AR gene results in the lack of the α1b-AR protein, which is reflected by the decrease of total α1-AR binding sites in various tissues of the α1b−/− mice. On the other hand, the expression of the α2 and β-AR was not significantly different between the α1b +/+ and −/− mice as indicated by ligand binding studies using [3H]RX821002 and [125I]cyano pindolol in cerebellum and heart, respectively (the α2 and β-AR ranged from 150 to 180 and from 30 to 35 fmol/mg of protein, respectively, for both genotypes) (results not shown).
To better assess the expression of different α1-AR subtypes, competition binding experiments using 5-methylurapidil were performed in some tissues of α1b +/+ and −/− mice. The affinity of 5-methylurapidil for the α1-AR subtypes from different species is high (Ki ≈10−9 M) for the α1a and low (Ki ≈10−7 M) for the α1b as well as for the α1d AR (3). In agreement with these findings, 5-methylurapidil displayed low affinity (Ki ≈2 × 10−7 M) also for the mouse α1b-AR expressed in COS-7 cells (results not shown). Thus, competition binding experiments with 5-methylurapidil should allow us to discriminate the α1a-AR (measured as high-affinity binding sites) from the α1b and α1d-AR (measured together as low-affinity binding sites).
The monophasic low-affinity competition curve of 5-methylurapidil in the liver of the α1b+/+ mice strongly suggests the large prevalence of the α1b-AR in this tissue. This is in agreement with the almost complete loss of α1-AR binding sites in the α1b−/− mice (Table 1). On the other hand, the prevalence of high-affinity binding sites for 5-methylurapidil in the kidney of the α1b+/+ mice suggests that in this tissue the α1a-AR is the most abundant subtype. This also is in agreement with the observation that the α1b−/− mice do not display any significant loss of total receptors in the kidney (Table 1). In both cerebral cortex and cerebellum of the α1b+/+ mice the biphasic competition curves of 5-methylurapidil suggest the coexistence of the α1a-AR with one or both of the other subtypes. In the α1b−/− mice, a selective decrease of the low-affinity sites in cerebral cortex and cerebellum (Table 2) reflects the loss of the α1b-AR with no change in the α1a-AR number. On the other hand, the remaining low-affinity binding sites in both cerebral cortex and cerebellum of the α1b−/− mice (Table 2) might reflect the presence of the α1d-AR in these tissues.
The results of the ligand binding studies are in agreement with the mRNA levels as tested by RT-PCR. The presence of all three α1-AR subtype mRNA transcripts in brain (Fig. 2) agrees with the biphasic displacement curves of 5-methylurapidil in cerebral cortex and with the selective decrease of the low-affinity binding component in the α1b−/− mice (Table 2). However, the abundance of each α1-AR subtype in various tissues could have not been predicted from the results of the RT-PCR. For example, the important loss of α1-AR binding sites in the heart of the α1b−/− mice indicates that in this organ the number of α1b-ARs is much greater than predicted from the RT-PCR studies, which detected apparently similar amounts of the three α1-AR mRNA.
Measurement of Blood Pressure in αib +/+ and −/− Mice.
The large decrease of α1-AR in the heart and the loss of the α1b-AR mRNA in the aorta of the α1b−/− mice prompted us to compare the cardiovascular regulation in α1b +/+ and −/− mice. Male mice between 12–18 weeks of age were sacrificed and analyzed for their heart weight/body weight ratios, which did not significantly differ between α1b +/+ and −/− mice (mean ± SE of 10 mice: 5.6 ± 0.5 and 5.1 ± 0.1 mg/g for α1b +/+ and −/−, respectively). Under basal conditions, either heart rate and blood pressure values were similar in the two groups of mice (mean ± SE of 20 mice: heart rate 551 ± 25 and 479 ± 20 bpm; mean arterial blood pressure 119.3 ± 6.6 and 118 5 ± 6.4 mmHg for α1b +/+ and −/−, respectively). Increasing doses of phenylephrine progressively increased the blood pressure over basal in both α1b +/+ and −/− mice.
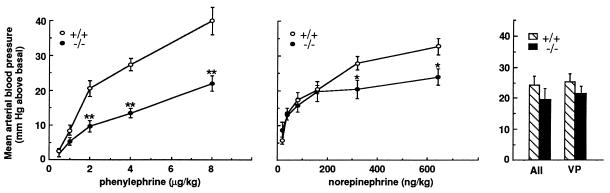
As shown in Fig. 3, the blood pressure response induced by increasing doses of phenylephrine was considerably reduced in the α1b −/− as compared with the +/+ mice. The maximal dose of phenylephrine used increased the blood pressure above basal by 40 mmHg in the α1b +/+, but only by 22 mmHg in the −/− mice. The effect induced by 2 μg/kg of phenylephrine was almost completely inhibited in mice of both genotypes by the coadministration of two α1-antagonists REC2739 and 3016 (22) administered intravenously at the dose of 10 μg/kg each 30 min before the agonist (results not shown). This supports the notion that the phenylephrine-induced response in vivo is mainly α1-adrenergic.
Figure 3.
Blood pressure response in α1b +/+ and −/− mice. Due to the dysrythmic properties of high doses of phenylephrine and norepinephrine, absolute maximal responses were not attempted. The results are the mean ± SE of 10 dose-response curves for each genotype. For angiotensinII (AII, 50 ng/kg) and vasopressin (VP, 30 milliunits/kg) the results are the mean ± SE from six mice of each genotype. ∗, P < 0.05 and ∗∗, P < 0.001 as compared with α1b+/+.
Despite the diminished response to phenylephrine in α1b−/− mice, the increase of blood pressure induced by angiotensinII or vasopressin did not differ significantly between the α1b +/+ and −/− mice (Fig. 3). Altogether, these findings provide strong evidence that the decreased blood pressure response in the α1b−/− mice is truly resulting from the knockout of the α1b-AR.
Similarly to what was observed for phenylephrine, the blood pressure response induced by the natural agonist norepinephrine in the α1b −/− mice also was reduced as compared with that observed in the +/+ mice. However, the reduction in the blood pressure response in α1b−/− mice was significant only for the higher doses of norepinephrine tested and overall smaller than that observed for phenylephrine. This might be explained by the fact that the blood pressure response induced by norepinephrine results from multiple mechanisms via the activation of different ARs, whereas the effect of phenylephrine is almost exclusively mediated by the α1AR. Thus, the lack of the α1b-AR in α1b−/− mice has a more pronounced effect on the blood pressure response induced by α1-selective phenylephrine than by norepinephrine.
Aorta Contractility in αib +/+ and −/− Mice.
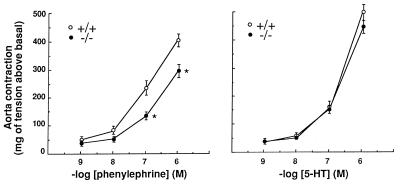
To assess the functional consequences due to the loss of the α1b-AR mRNA in the aorta of α1b−/− mice, we measured the effect of the α1-agonist phenylephrine on the contraction of isolated aortic rings from male α1b +/+ and −/− mice. Phenylephrine induced a concentration-dependent contractile response of the aortic rings of α1b+/+ mice ranging from 42 to 421 mg of tension above basal at 10−9 and 10−6 M phenylephrine, respectively (Fig. 4).
Figure 4.
Aorta contractility in α1b +/+ and −/− mice. The results are the mean ± SE of 13 concentration-response curves for each genotype and for both phenylephrine and serotonin (5-HT). Concentrations of phenylephrine higher than 10−6 M did not further increase, but rather decreased the contractions of the aortic rings derived from both α1b +/+ and −/− mice. ∗, P < 0.05 as compared with α1b+/+.
Interestingly, the efficacies of the contractile responses to 10−7 and 10−6 M phenylephrine were significantly reduced of 40% and 28%, respectively, in aortic rings from α1b −/− as compared with +/+ mice. On the other hand, the contractile effect induced by increasing concentrations of serotonin was similar in α1b +/+ and −/− mice (Fig. 4). The effect of 10−7 M phenylephrine was completely inhibited by the α1-antagonist prazosin (10−6 M) in both α1b +/+ and −/− mice (results not shown). In addition, stimulation with the α2-agonist UK14,304 (10−6 M) did not induce any significant increase in tension, thus excluding the involvement of an α2-AR in the mouse aorta contractile response (results not shown). An involvement of the α2-AR in the murine aortic contraction has been recently ruled out using different antagonists (23). Altogether these findings provide strong evidence that the contractile effect of phenylephrine in mouse aorta is α1-adrenergic and that the α1b-AR contributes to mediate the aortic contractions induced by α1 agonists. This is in agreement with recent findings suggesting that, despite the apparently predominant role of the α1d-AR in a number of rat vessels, vascular contractility cannot be mediated by a single receptor subtype (24, 25).
DISCUSSION
In this study, we describe the targeted inactivation of the mouse α1b-AR gene and its consequences on the blood pressure response. Our findings provide strong evidence that the α1b-AR is a mediator of the blood pressure response and of the aorta contractility induced by α1 agonists. This work contributes to unravel the functional role in vivo of different α1-AR subtypes for which a knockout has not been described so far.
Cardiovascular Implications of the αib-AR Knockout.
Clinical efficacy of α1 antagonists as antihypertensive drugs reflects the important physiological role of α1-ARs in vascular function and in the maintenance of arterial blood pressure. Thus, this study was exclusively focused on the functional characterization of the α1b-AR knockout model on the in vivo blood pressure response and in vitro vascular contractility.
The fact that the increase of the mean arterial blood pressure induced by phenylephrine is reduced by ≈45% in α1b −/− as compared with +/+ mice suggests that the α1b-AR can mediate a large portion of the vasopressor response to α1 agonists. The blood pressure response mediated by the α1b-AR might involve, at least in part, its effect on the control of the vascular tone. This is supported by the observation that in α1b −/− the aorta contractility induced by phenylephrine also was reduced as compared with the +/+ mice. Future studies will attempt to investigate the role of the α1b-AR in the contractility of other small resistance mouse vessels that might be more directly involved in the regulation of blood pressure.
The role of the α1b-AR in the control of the vascular tone was not clearly anticipated by previous pharmacological studies. In vivo studies on blood pressure responses in various species have been difficult to interpret because the available pharmacological agents have limited selectivity for a single α1-AR subtype and some of them have additional properties. Recent studies suggest a predominant role of the α1d-AR in the vascular contractions induced by α1 agonists in the rat (24). However, the profile of the α1d selective antagonist BMY7378 in antagonizing norepinephrine-induced contractions does not suggest competitive antagonism at a single receptor (25). Thus, even in isolated vessels the results deriving from the use of selective antagonists might be difficult to interpret. Therefore, the α1b-AR knockout represents an useful model to further investigate the properties of α1a- and α1d-selective drugs in an α1b-lacking background.
The important decrease of α1-AR binding sites in the heart of the α1b−/− mice (Table 1) suggests that the α1b-AR plays an important functional role in cardiac function. The observations that the heart weight/body weight ratios as well as the basal values of both heart rate and mean arterial blood pressure do not differ between α1b +/+ and −/− mice seem to exclude a major role of the α1b-AR on the normal development and function of the heart. However, the role of the α1b-AR in catecholamine-induced increase of cardiac inotropy remains to be investigated with direct measurements of the heart contractile function in α1b +/+ and −/− mice. At present, we cannot exclude that the role of the α1b-AR in mediating the blood pressure response to phenylephrine involves both vascular and cardiac mechanisms. Interestingly, overexpression of a constitutively active α1b-AR mutant in the heart of transgenic mice resulted in cardiac hypertrophy with increased heart weight/body weight ratios (26). These findings suggest that the α1b-AR can activate biochemical mechanisms that contribute to the development of cardiac hypertrophy and related cardiac diseases. This also is supported by in vitro studies on cardiomyocyte systems showing that Gq-coupled receptors may mediate cardiac hypertrophy (27, 28). In future studies, it might be interesting to investigate whether the inactivation of the α1b-AR can protect mice from the development of the cardiac hypertrophy induced by pressure overload or other conditions.
Although our data clearly demonstrate that the α1b-AR can participate in the regulation of vasoconstriction and hence blood pressure, we did not observe alterations of basal blood pressure in the knockout mice. This apparent discrepancy may be explained by several mechanisms. First, nonadrenergic mechanisms, e.g., the renin-angiotensin or nitric oxyde systems, may compensate for the potential decrease of a tonic response. Second, it should be considered that our blood pressure experiments were performed in conscious mice, i.e., most likely under conditions of low sympathetic tone. Under these experimental conditions, the contribution of any AR to blood pressure maintenance will be underestimated by measurements of basal blood pressure. Future studies with hemodynamic stress models may be more informative in this respect.
The finding that α1b-AR knockout mice displayed a decreased blood pressure response to α1 agonists was not expected because of the potential compensatory effects of the other α1-AR subtypes. Our results suggest that, despite the presence of multiple α1-AR subtypes in the same tissue, only partial functional redundancy is at the cardiovascular level, i.e., multiple α1-AR subtypes can mediate the vasopressor response, but cannot compensate each other. This has important implications for better understanding the cardiovascular effects of drugs acting at the α1-AR and for more precisely defining the goals linked to the development of α1-AR subtype-selective ligands.
Conclusions.
In this study, our characterization of the α1b-AR knockout model has focused on the cardiovascular system. Future studies will extend the functional investigation to other organs of the knockout mice in which the α1b-AR normally is expressed. The α1b-AR knockout model provides a useful tool to elucidate the functional specificity of different α1-AR subtypes and to further elucidate the pharmacological effects of adrenergic drugs. A full understanding of the functional implications of AR heterogeneity awaits the knockout of all AR subtypes and the intercross among these different knockout models.
This study also might provide a contribution to the investigation of the mechanisms involved in the control of blood pressure and its disregulation. Human hypertension is caused by the interplay of several “risk” genes and environmental factors. An important line of investigation in hypertension concerns the mechanisms of integration among different homeostatic systems, including the sympathoadrenomedullary, the renin-angiotensin, the nitric oxide as well as those involved in the control of sodium balance. It could be envisaged that intercrosses between the α1b-AR knockout and other mouse models mutated in various genes potentially involved in the control of blood pressure might contribute to investigate the role of the sympathoadrenomedullary system in hypertension.
Table 1.
Ligand binding in tissues of α1b+/+ and −/− mice
| Tissue | α1b+/+
|
α1b−/−
|
||
|---|---|---|---|---|
| Bmax, fmol/mg protein | % high affinity | Bmax, fmol/mg protein | % high affinity | |
| Cerebral cortex | 94.3 ± 4.7 | 42.9 ± 2.1 | 54.0 ± 3.3* | 80.2 ± 3.7* |
| Cerebellum | 76.7 ± 5.6 | 60.8 ± 1.2 | 51.8 ± 3.0* | 90.7 ± 1.5* |
| Kidney | 22.6 ± 1.5 | 76.2 ± 3.3 | 21.2 ± 1.2 | 86.1 ± 4.5* |
| Liver | 39.4 ± 9.6 | 0 | 0.6 ± 0.2* | n.d. |
| Heart | 4.7 ± 1.1 | n.d. | 1.2 ± 0.3 | n.d. |
[3H] prazosin binding in saturation and competition experiments was measured as described in Materials and Methods. Receptor number in heart is lower than reported in other studies (26) probably because a crude membrane preparation has been used. The Ki high and Ki low of 5-methylurapidil ranged 0.5–1 and 80–200 nM in various tissues, respectively. The results are the mean ± S.E. of 4–7 independent experiments. n.d., not determined. ∗, P < 0.05 as compared to α1b+/+ mice in a paired two-tailed t test.
Table 2.
Pharmacological profile of the α1−AR subtypes in tissues of the α1b+/+ and −/− mice
| Tissue | α1b+/+
|
α1b−/−
|
||
|---|---|---|---|---|
| High affinity, fmol/mg protein | Low affinity, fmol/mg protein | High affinity, fmol/mg protein | Low affinity, fmol/mg protein | |
| Cerebral cortex | 40 | 54 | 43 | 11 |
| Cerebellum | 47 | 30 | 47 | 5 |
| Kidney | 17 | 6 | 18 | 3 |
| Liver | 0 | 39 | n.d. | n.d. |
The binding sites (fmol/mg of protein) displaying high- and low-affinity for 5-methylurapidil have been calculated from the results shown in Table 1. n.d., not determined.
Acknowledgments
We thank Dr. A. Leonardi and Dr. R. Testa from Recordati, Milan, Italy for their gift of the compounds REC2739 and 3016 as well as for helpful suggestions. This work was supported by grants from the Fonds National Suisse de la Recherche Scientifique to S.C. (31-33684.92) and from the Deutsche Forschungsgemeinschaft to M.C.M.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: AR, adrenergic receptor; RT-PCR, reverse transcription–PCR; ES, embryonic stem cell.
Data deposition: The sequence reported in this paper has been deposited in the EMBL database (accession no. Y12738).
References
- 1.Ruffolo R R, editor. The α1-Adrenergic Receptors. Clifton, NJ: Humana; 1987. [Google Scholar]
- 2.Minneman K P, Esbenshade T A. Annu Rev Pharmacol Toxicol. 1994;34:117–133. doi: 10.1146/annurev.pa.34.040194.001001. [DOI] [PubMed] [Google Scholar]
- 3.Michel M C, Kenny B, Schwinn D A. Naunyn-Schmiedeberg’s Arch Pharmacol. 1995;352:1–10. doi: 10.1007/BF00169183. [DOI] [PubMed] [Google Scholar]
- 4.Graham R M, Perez D M, Hwa J, Piascik M T. Circ Res. 1996;78:737–749. doi: 10.1161/01.res.78.5.737. [DOI] [PubMed] [Google Scholar]
- 5.Link R E, Stevens M S, Kulatunga M, Scheinin M, Barsh G S, Kobilka B K. Mol Pharmacol. 1995;48:48–55. [PubMed] [Google Scholar]
- 6.Susulic V S, Frederich R C, Lawitts J, Tozzo E, Kahn B B, Harper M E, Himms-Hagen J, Flier J S, Lowell B B. J Biol Chem. 1995;270:29483–29492. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- 7.MacMillan L B, Hein L, Smith M S, Piascik M T, Limbird L E. Science. 1996;273:801–803. doi: 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- 8.Rohrer D K, Desai K H, Jasper J R, Stevens M E, Regula D P, Jr, Barsh G S, Bernstein D, Kobilka B K. Proc Natl Acad Sci USA. 1996;93:7375–7380. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotecchia S, Schwinn D A, Randall R R, Lefkowitz R J, Caron M G, Kobilka B K. Proc Natl Acad Sci USA. 1988;85:7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier B C. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 11.Konecki D S, Brennand J, Fuscoe J C, Caskey C T, Chinault A C. Nucleic Acids Res. 1982;10:6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso-Llamazares A, Zamanillo D, Casanova E, Ovalle S, Calvo P, Chinchetru M A. J Neurochem. 1995;65:2387–2392. doi: 10.1046/j.1471-4159.1995.65062387.x. [DOI] [PubMed] [Google Scholar]
- 13.Schwinn D A, Lomasney J W, Lorenz W, Szklut P J, Fremeau R T, Yang-Feng T L, Caron M G, Lefkowitz R J, Cotecchia S. J Biol Chem. 1990;265:8183–8189. [PubMed] [Google Scholar]
- 14.Lomasney J W, Cotecchia S, Lorenz W, Leung W-Y, Schwinn D A, Yang-Feng T L, Brownstein M, Lefkowitz R J, Caron M G. J Biol Chem. 1991;266:6365–6369. [PubMed] [Google Scholar]
- 15.Michel M C, Büscher R, Kerker J, Kraneis H, Erdbrügger W, Brodde O E. Naunyn-Schmiedeberg’s Arch Pharmacol. 1993;348:385–395. doi: 10.1007/BF00171338. [DOI] [PubMed] [Google Scholar]
- 16.Wiesel P, Mazzolai L, Nussberger J, Pedrazzini T. Hypertension. 1997;29:1025–1030. doi: 10.1161/01.hyp.29.4.1025. [DOI] [PubMed] [Google Scholar]
- 17.Fluckiger J P, Gremaud G, Waeber B, Kulik A, Ichino A, Nussberger J, Brunner H R. J Appl Physiol. 1989;67:250–255. doi: 10.1152/jappl.1989.67.1.250. [DOI] [PubMed] [Google Scholar]
- 18.Lembo G, Iaccarino G, Vecchione C, Rendina V, Trimarco B. Hypertension. 1995;26:290–293. doi: 10.1161/01.hyp.26.2.290. [DOI] [PubMed] [Google Scholar]
- 19.Ramarao C S, Denker J M, Perez D M, Gaivin R J, Riek R P, Graham R M. J Biol Chem. 1992;267:21936–21945. [PubMed] [Google Scholar]
- 20.Weinberg D H, Trivedi P, Tan C P, Mitra S, Perkins-Barrow A, Borkowski D, Strader C D, Bayne M. Biochem Biophys Res Com. 1994;201:1296–1304. doi: 10.1006/bbrc.1994.1845. [DOI] [PubMed] [Google Scholar]
- 21.Perez D M, Piascik M T, Graham R M. Mol Pharmacol. 1991;40:876–883. [PubMed] [Google Scholar]
- 22.Leonardi A, Testa R, Motta G, De Benedetti P G, Hieble P, Giardina D. Perspect Recept Res. 1996;24:135–152. [Google Scholar]
- 23.Michel M C, Chen H, Fetscher C, Bischoff A. Pharmacologist. 1997;39:44. (abstr.). [Google Scholar]
- 24.Piascik M T, Guarino R D, Smith M S, Soltis E E, Saussy D L, Jr, Perez D M. J Pharmacol Exper Ther. 1995;275:1583–1589. [PubMed] [Google Scholar]
- 25.Kenny B A, Chalmers D H, Philpott P C, Naylor A M. Br J Pharmacol. 1995;115:981–986. doi: 10.1111/j.1476-5381.1995.tb15907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milano C A, Dolber P C, Rockman H A, Bond R A, Venable M E, Allen L F, Lefkowitz R J. Proc Natl Acad Sci USA. 1994;91:10109–10113. doi: 10.1073/pnas.91.21.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shubeita H E, McDonough P M, Harris A N, Knowlton K U, Glembotski C C, Brown J H, Chien K R. J Biol Chem. 1990;265:20555–20562. [PubMed] [Google Scholar]
- 28.Glembotski C C, Irons C E, Krown K A, Murray S F, Sprenkle A B, Sei C A. J Biol Chem. 1993;268:20646–20652. [PubMed] [Google Scholar]