Abstract
There is a clinical need for a tissue engineered vascular graft (TEVG), and combining stem cells with biodegradable tubular scaffolds appears to be a promising approach. The goal of this study was to characterize the incorporation of muscle derived stem cells (MDSCs) within tubular polyester urethane urea (PEUU) scaffolds in-vitro to understand their interaction, and to evaluate the mechanical properties of the constructs for vascular applications. Porous PEUU scaffolds were seeded with MDSCs using our recently described rotational vacuum seeding device, and cultured inside a spinner flask for 3 or 7 days. Cell viability, number, distribution and phenotype were assessed along with the suture retention strength and uniaxial mechanical behavior of the TEVGs. The seeding device allowed rapid even distribution of cells within the scaffolds. After 3 days, the constructs appeared completely populated with cells that were spread within the polymer. Cells underwent a population doubling of 2.1-fold, with a population doubling time of 35 hrs. Stem cell antigen-1 (Sca-1) expression by the cells remained high after 7 days in culture (77 ± 20% vs 66±6% at day 0) while CD34 expression was reduced (19 ± 12% vs 61±10% at day 0) and myosin heavy chain expression was scarce (not quantified). The estimated burst strength of the TEVG constructs was 2127±900 mmHg and suture retention strength was 1.3±0.3 N. We conclude from this study that MDSCs can be rapidly seeded within porous biodegradable tubular scaffolds while maintaining cell viability and high proliferation rates and without losing stem cell phenotype for up to 7 days of in-vitro culture. The successful integration of these steps is thought necessary to provide rapid availability of TEVGs, which is essential for clinical translation.
Introduction
Cardiovascular disease is the leading cause of death in the western world, claiming almost one million lives every year in the United States alone [1]. Most procedures used to alleviate cardiovascular disease involve surgical implantation of vascular graft, including coronary artery by-pass for myocardial revascularization, peripheral by-pass for limb revascularization and arteriovenous fistulae for dialysis. In the United States, over one million vascular procedures are performed each year involving small diameter vessels [2]. Limited availability of autologous vessels and inadequate performance of synthetic grafts in small diameter (< 5mm.) vascular replacement make current alternatives suboptimal [3-7]. The fabrication of a tissue-engineered vascular graft (TEVG) appears to hold great promise as alternative conduits [8, 9].
Different approaches to achieve a fully functional TEVG have been reported, including a completely cellular approach [10, 11], use of decellularized matrices [12, 13], and a combination of cells and either natural or synthetic scaffolds [8, 14]. In order to achieve clinical success, there are several criteria that must be met by a TEVG, regarding both mechanical and biological properties [15]. Most TEVG approaches that have been developed to date have been hampered by thrombosis, poor levels of remodeling, inadequate mechanical properties, and a lack of a vasomotor response [8]..
Most vascular tissue engineering approaches have relied on some form of scaffold to provide mechanical integrity to a TEVG so that it will not rupture upon implantation to the arterial circulation [15, 16]. Synthetic biodegradable polymers are particularly promising due to the ability to control dimensions and mechanical properties of the material [9, 17].
It is thought that in order to be clinically viable, a vascular tissue engineering approach should utilize autologous cells incorporated into the scaffold [15]. Additionally, the cell source should be easy to isolate and expand in-vitro to reduce the time of construction. While many previous approaches have prompted the use of terminally differentiated smooth muscle (SMCs) and/or endothelial cells, these have often shown an inability to reconstitute tissues [18]. Alternative autologous cells for tissue engineering applications that have shown promise are multi-potent progenitor cells that have been identified in adult tissues [19-25]. Progenitor cells are capable of differentiating into several different hematopoietic and mesenchymal lineages[26-29] and have recently been shown to have potential in several clinical applications including cardiovascular, hematopoietic, and osteoarticular disorders [30-33]. In clinical vascular medicine, a recent study showed the successful implantation of a TEVG in pediatric patients using a biodegradable scaffold and bone marrow progenitor cells [34].
The manner by which the cells are incorporated inside the scaffold can also be an important determinant for the feasibility of constructing a clinically viable TEVG [35]. Seeding requirements for three-dimensional scaffolds include a high yield to maximize the utilization of donor cells, and a spatially-uniform distribution of viable cells to provide a basis for uniform tissue regeneration [36]. Most current approaches have relied either on static culture of the construct within a cell suspension or dynamic intraluminal seeding within complex bioreactors [14, 37, 38]. We have recently reported the development of a seeding device for tubular tissue engineered structures that can efficiently incorporate a large number of cells in a short period of time with an even distribution throughout the thickness [39].
The goal of this study was to combine a porous biodegradable elastomeric scaffold with muscle-derived stem cells (MDSC) using our novel rotational vacuum seeding technique to achieve cellularized tubular constructs in a short period of time [39].
Materials and methods
Polymer synthesis and scaffold preparation
Poly(ester urethane)urea (PEUU) based on polycaprolactone diol (PCL, MW=2000), 1,4-diisocyanatobutane (BDI, Fluka) and putrescine was synthesized as described previously using a two-step solution polymerization [17]. Briefly, synthesis was carried out in a 250 mL round-bottom flask under dry nitrogen with reactant stoichiometry of 2:1:1 (BDI: PCL: putrescine). BDI at 15 wt% in Dimethyl Sulfoxide (DMSO) was continuously stirred with 25 wt% PCL in DMSO followed by stannous octoate addition. The reaction was allowed to proceed for 3 h at 80°C followed by cooling at room temperature. Putrescine was then added drop wise with stirring and the reaction was continued at room temperature for 18 hours. The resulting PEUU solution was precipitated in distilled water, the wet polymer was immersed for 24 hours in isopropanol to remove unreacted monomers, and the polymer was dried under vacuum at 50°C for 24 hours.
PEUU tubular scaffolds (length = 2 cm) were fabricated by thermally induced phase separation (TIPS), using a previously described method [40]. Briefly, PEUU was dissolved in DMSO to form a 10% solution. The solution was then injected into a cylindrical mold consisting of an outer glass tube (inner diameter 5.5 mm) and an inner polytetrafluoroethylene (PTFE) cylinder (outer diameter 4.5 mm), coaxially fixed by two rubber stoppers. The mold was cooled to a temperature of −80°C for 3 hours and was then removed and placed into absolute alcohol at temperature 4°C for 7 days to extract the DMSO. The alcohol was changed daily. The scaffold was immersed in water for 2 days and then freeze-dried for 24 hours. After removal of the scaffolds from the mold, internal diameter (ID) and wall thickness were measured with a Vernier caliper.
Cell source and culture
Mouse MDSCs were isolated by means of an established, previously described, pre-plating technique[23]. Cells were then plated at low density (200 cells/cm2) on a 175 cm2 flask and cultured at 37° C and 5% CO2 with complete Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (Atlanta Biologicals; Norcross, GA), 10% horse serum (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were expanded to the desired number and were only used between passages 10−15. Media changes were performed every 48 hours during culture. Before use, MDSC monolayers were washed three times in Dulbecco's modified phosphate buffered saline (DPBS) and then incubated with 0.1% trypsin for 5 minutes to remove them from the flasks. MDSCs were then centrifuged at 1200 rpm for 5 minutes to form a pellet, and then resuspended in DMEM to the desired concentration in preparation for seeding.
Cell seeding and TEVG culture
In order to incorporate the MDSCs within the scaffold, a previously described rotational vacuum seeding device was utilized [39]. Briefly, an airtight chamber was designed to hold two coaxial tees in rotation along the longitudinal axis using an electrical motor (100−200 rpm). The constructs (n = 12) were mounted inside the chamber between the two tees and simultaneously perfused with 5 ml of cell suspension (2 × 106 cells/ml) by means of a precision syringe pump with a steady rate of 5 ml/min. During the process a constant relative negative pressure was maintained within the chamber to facilitate transmural flow across the construct (Fig. 1). The constructs were then flushed with 5 ml of plain DMEM in order to wash residual cells from the lumen of the scaffolds, removed and incubated in a Petri dish for 1 hour.
Figure 1.
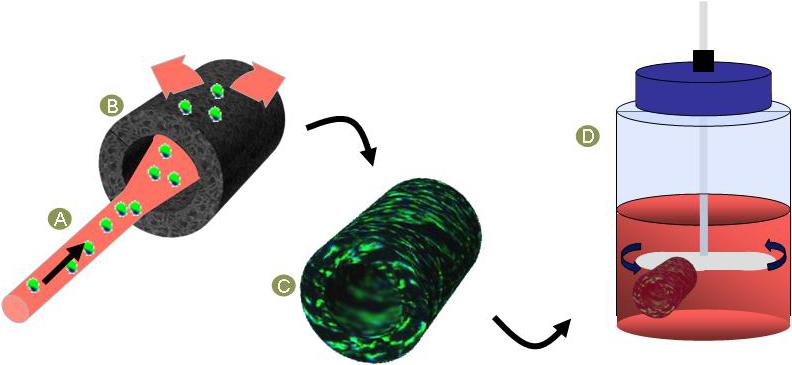
Principle of action of seeding technique (39) and dynamic culture. The seeding solution was transluminally infused (A) into a rotating porous scaffold (B), yielding a seeded TEVG construct (C). Spinner flask culture rotating at 15 rpm was used to enhance nutrients diffusion (D).
After seeding, the TEVGs were placed in 500 ml spinner flasks (196580575, Bellco Glass Inc, NJ) with 100 ml of culture media supplemented with 50 μg/ml of ascorbic acid and stirred at 15 rpm for 7 days (Fig. 1). Control constructs were cultured either within the spinner flasks without ascorbic acid or in static culture in a Petri dish.
Cell distribution and TEVG morphology
To assess cell spreading and distribution, ring segments of different areas of each construct were washed in DPBS, fixed with 2% paraformaldehyde, and embedded in cryomatrix (Thermoshandon Inc., Pittsburgh, PA). Sections (10μm) were obtained with a cryotome (Thermoshandon Inc., Pittsburgh, PA) and permeabilized with 0.1% Triton-X 100 solution in PBS for 10 minutes. Next, the samples were incubated with Alexa 488-conjugated phalloidin (Sigma Aldrich, St. Louis, MO) (dilution 1:250) for 60 minutes in a moist chamber to prevent drying of the samples. Unbound phalloidin was removed by subsequent washes in PBS. For nuclear visualization, cells were counter-stained with DAPI. Samples were mounted in gelvatol and viewed at 200× under fluorescent optics using a Nikon Eclipse E600 microscope (Nikon, Melville, NY).
To assess collagen production and TEVG histology, separate ring segments were fixed in 10% neutral buffered formalin for 1 hour. They were then embedded in paraffin blocks and 5 μm sections were cut using a microtome (Thermo Shandon Inc., Pittsburgh, PA). Sections were mounted on slides, stained with Masson's trichrome and viewed under bright light optics using a Nikon Eclipse E600 microscope. Collagen production was qualitatively assessed on acquired images using Adobe Photoshop (v. 7.0, Adobe Systems Inc, USA).
Proliferation (MTT assay)
To assess proliferation and viability within the constructs, samples were analyzed with MTT mitochondrial activity assay at days 1, 3 and 7. [41] Briefly, before the TEVG was fixed, three rings of approximately equal size (normalized by weight) were randomly sectioned from each construct and placed in the wells of a 96-well plate with 200 μl of serum free α-MEM and 20 μl of Thyazolyl Blue Tetrazolium Bromide (Sigma Aldrich, St. Louis, MO). Samples were then incubated at 37°C for 4 hours to allow crystal formation. The supernatant volume was carefully removed and 200 μl of 0.04 N HCl in 2-propanol solution was added to dissolve the crystals. Samples were kept in the dark at 4°C for 24 hours. Finally, absorbance readings were taken for 100 μl of the solution at a wave length of 550 nm using a microplate reader (Bio Rad, Hercules, CA). The final number of cells was calculated using a standard curve generated for previously known cell concentrations and transforming absorbance to cell number using the equation generated by the slope of the curve. The average reading of the three rings was used as the result for each construct.
Comparisons between groups (ascorbic acid supplemented and non-supplemented constructs) were made by a two-tailed paired t-test and results were expressed as difference in cell number. Population doubling time (PDT) and number of population doublings (PD) were calculated from those values as: PD=log(cell number at day 3/cell number at day 1), PDT=Time/PD.
Stem cell characterization
Cells were characterized at day 0 (seeding day) and after 7 days of TEVG culture by flow cytometry for CD34 and Sca-1 expression. Briefly, TEVGs were incubated with 0.1% trypsin for 5 minutes to remove MDSCs from the scaffolds. The cells were then pelleted and blocked with 10% mouse serum for 15 minutes. Some cells were then labelled with rat anti-mouse Sca-1 (phycoerythrin (PE) anti-mouse Ly6A, 1 μl stock, 553336, Pharmingen, USA) and CD34 (biotinylated, 1 μl stock Purified Rat Anti-Mouse CD 34(1HC)) monoclonal antibodies for 15 minutes. The same proportion of cells were treated with equivalent amounts of isotype control antibodies PE-Mouse IgG 2b (33805×, Pharmingen, USA) and biotin purified Rat IgG (11021D, Pharmingen, USA). Both fractions then were washed with PBS and labelled with streptavidinallophycocyanin (APC, 1:300, 13049A, Pharmingen, USA). 7-amino-actinomycin D (7-ascorbic acid D) or Via-Probe (555816, Pharmingen, USA) was added to exclude nonviable cells from the analysis. Appropriate gating was performed to determine Sca-1 and CD34 expression via flow cytometry with a FACS Aria (Becton Dickinson, San Jose, CA). Cells at day 0 were processed following the same protocol.
Immunofluorescence was performed to corroborate Sca-1 expression and to assess myotube formation (fusion) by myosin heavy chain (MHC) expression. Briefly, frozen sections were obtained as described above and incubated with 0.1% Triton-X 100 in PBS for 10 minutes. Non-specific binding of antibodies was blocked by incubating the samples for 45 minutes with 5% normal donkey serum in PBS with 0.5% bovine serum albumin (Fraction V, Sigma-Aldrich, St. Louis, MO) and 0.15% glycine (Sigma-Aldrich, St. Louis, MO). Following this, the sections were incubated at room temperature with the primary antibodies (Sca-1 (1:500) and MHC (1:500) [Sigma-Aldrich, St. Louis, MO]) diluted in blocking solution for 60 minutes. Unbound primary antibody was removed by subsequent washes in PBS. Next, the samples were incubated with a Cy3-conjugated (Sigma-Aldrich, St. Louis, MO) secondary antibody (1:500) for 1 hr at room temperature and then rinsed 3 times for 15 minutes with PBS. For nuclear visualization, cells were counter-stained with DAPI. The samples were then mounted in gelvatol and viewed under confocal microscopy using an Olympus F1000 confocal microscope. Positive controls were MDSCs cultured at high density for 7 days with low serum. Under these conditions, MDSCs undergo myogenic differentiation readily [42].
Mechanical properties
To measure uniaxial mechanical properties of the scaffolds, a tensile tester (Tytron™250, MTS System Corp., Minneapolis, MN) mounted with a 10 lb force transducer (Model 661.11B-02, MTS System Corp., Minneapolis, MN) was used. Dry scaffolds were cut into rings (width ∼ 3 mm) and cut open to obtain a long, flat sample oriented in the circumferential direction of the scaffolds. Adjacent samples were cut in paraffin blocks and imaged under a microscope for measurement of the thickness of each scaffold with image-based techniques. Each specimen was mounted in the tensile system clamps, and specimen length and width were measured with a dial caliper. The specimens were pulled at 10 mm/min crosshead speed (strain rate ∼ 2.5%/sec) until rupture after 10 cycles of preconditioning at 20% strain. Measured load–displacement data were used to calculate Cauchy stress–strain by assuming an incompressible material [43]. Ultimate tensile stress (UTS) and strain to failure (STF) were taken as the maximum stress value before failure and its corresponding value of strain, respectively,. Burst pressure was estimated from UTS as done previously[44] by rearranging the law of Laplace; i.e.:
where:
D0 = Unpressurized internal diameter of the scaffolds
t = Initial thickness of the tested scaffolds
Suture retention testing was performed according to American National Standard Institute/Association for the Advancement of Medical Instruments (ANSI/AAMI) VP20 standards employing the same tensile testing apparatus used for the uniaxial testing. Briefly, each tubular scaffold was cut to obtain rectangular specimens (n = 5, length = 15 mm; width = 6mm) with the short edge of each specimen originally oriented circumferentially on the original tubular scaffold. A single loop of 5−0 PDS™ suture (Ethicon, Inc.) was created at approximately 2 mm from the short edge of each sample and secured to a hook connected to the clamp of the testing device. An extension rate of 2 mm/sec was used to pull the suture. Suture retention strength was taken as the maximum force recorded prior to pull-out of the suture.
Results
Tubular PEUU scaffolds were obtained with a pore size ranging from 20 μm in the outer layer to 200 μm in the inner layer. Final construct ID and wall thickness were approximately 4 mm and 300 μm respectively. Immediately after seeding, nuclear staining showed a high number of cells inside the constructs (Fig. 2-A). Distribution was even along the length and across the entire thickness of the TEVG. Macroscopically, the constructs showed a tissue-like structure (Fig. 2-B). After 3 days of dynamic culture, the constructs appeared completely populated with cells that were clearly spread throughout the scaffolds (Fig. 2-C). In contrast, the cells within the constructs that were maintained under static culture appeared to migrate from the center of the wall toward the inner and outer edges during this timeframe (Fig. 2-D).
Figure 2.
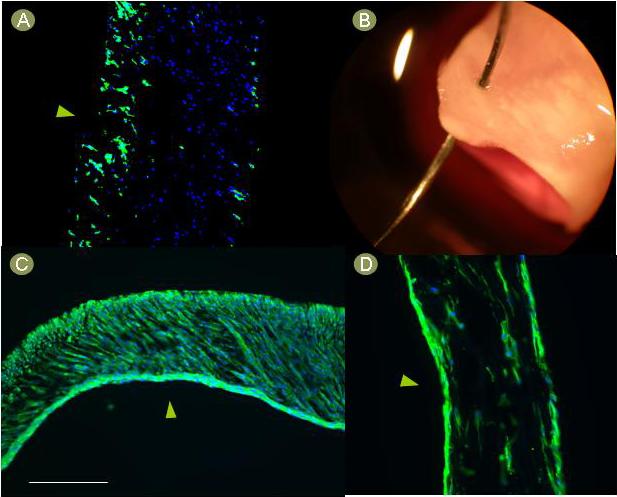
Macroscopic and microscopic aspect of MDSC-seeded TEVG. A) The constructs showed uniform transmural cellular distribution immediately after seeding. B) The macroscopic aspect after seeding resembled native tissue and had appropriate suturing features as demonstrated with a prolene 7.0 suture and a CV-1 needle. C) After 3 days of culture, the constructs were placed into spinner flasks, cells were spread through the entire thickness of the scaffolds. D) The static controls showed cell accumulation at the edges of the polymer. Green = phalloidin, blue = DAPI, arrowheads = lumen, scale bar = 100 um.
Proliferation analysis showed that the cells within the constructs supplemented with ascorbic acid in spinner flask culture exhibited a rapid proliferation starting from 116 ± 76 × 103 cells/mg (cell density per weight of construct) at day 0 and reaching 491 ± 145 × 103 at day 3 (n=3) (Fig. 3-A). The calculated number of PD was 2.1 while PDT was 35 hrs. A significant difference in proliferation was detected between the constructs supplemented with ascorbic acid and the non-supplemented controls. At day 1 the TEVGs supplemented with ascorbic acid had 174 ± 30 × 103 more cells/mg than those not supplemented (p<0.05, n=3), and at day 3 the difference was 121± 75 × 103 cells/mg (p<0.05, n=5) (Fig. 3-B).
Figure 3.
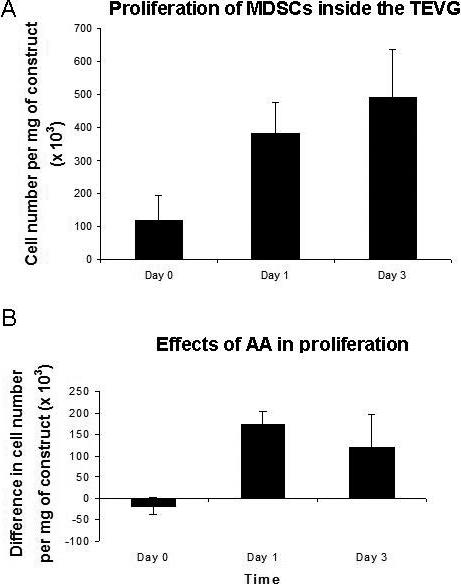
Proliferation analysis with MTT assay. A) The cells appeared viable and proliferated rapidly inside the constructs. B) Ascorbic acid enhanced proliferation in dynamic culture. The bars represent the differences in cell number between constructs supplemented with ascorbic acid and non-supplemented controls. The difference was significant both at day 1 (p < 0.05, n=3) and at day 3 (p < 0.05, n=5). The results are normalized by weight and the differences are expressed as means ± SD analyzed with a paired T-test.
The ascorbic acid supplemented TEVGs exhibited collagen production by day 7 while the non-supplemented constructs did not show any collagen production (Fig. 4).
Figure 4.
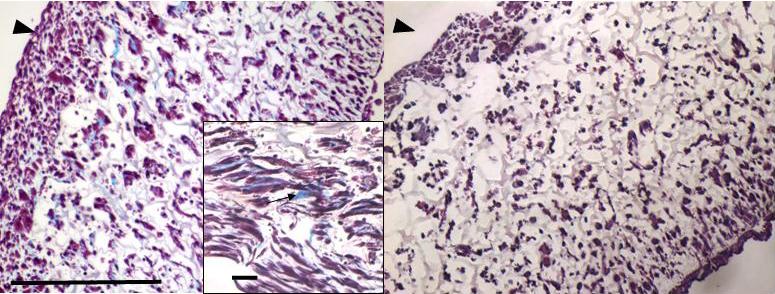
TEVG histology with Masson's trichrome. After 7 days, the constructs supplemented with ascorbic acid (left) had visible collagen deposition (black arrow on inset), differently from the controls (right) with no ascorbic acid. Blue = collagen, arrowheads = lumen, scale bar = 100um. Inset scale bar = 10 um.
The stem cell characterization at day 7 showed no significant difference in positive expression of Sca-1 in MDSCs,compared to that at day 0 (77 ± 20 % compared to 66 ± 6 %, respectively; n=3; p>0.05). Initial CD34 expression level was 61 ±10 % while at 7 days it was 19 ± 12% (n=3; p=0.011) (Fig. 5-A,B). Sca-1 positive expression by immunofluorescence was consistent with the flow cytometry analysis (Fig. 5-C). MHC expression was only scarcely noted in MDSCs after 7 days of culture (Fig. 5-D).
Figure 5.
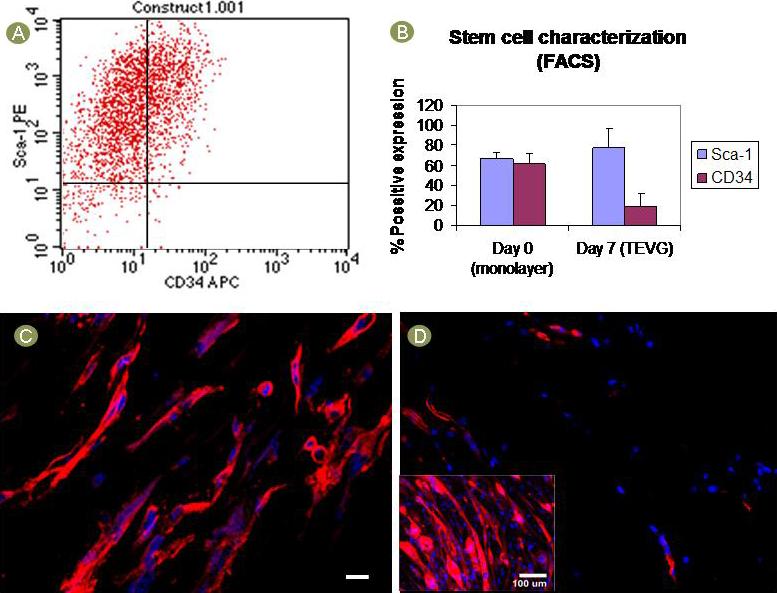
Stem cell characterization. A) Sca-1 and CD34 expression measured by flow cytometry. B) Sca-1 remained high and unchanged (p>0.05) while CD34 expression was decreased (p=0.011) after 7 days in culture. C) Sca-1 expression was confirmed by immunofluorescence. Red = sca-1, scale bar = 10um. D) MHC expression was scarce suggesting a low incidence of fusion and myotube formation. Positive control (inset) shows myotube formation of monolayer MDSCs. Red = MHC, blue = nuclei.
The measured UTS of the scaffold was 2.6±1.1 MPa (n=5), while the STF was 150±40%. PBurst was estimated to be 2127±900 mmHg. Suture retention strength was measured to be 1.3±0.3 N (n=5).
Discussion
In this alternative approach to the construction of a TEVG, we have successfully incorporated MDSCs within a synthetic biodegradable PEUU tubular scaffold by means of a novel vacuum and rotational seeding method. The cells were able to proliferate and populate the polymer while in dynamic culture, retaining their stem cell features, and producing collagen when stimulated with ascorbic acid. The seeding procedure was completed in less than 5 minutes and the scaffolds were completely populated in 3 days. Collagen deposits were evidenced after 7 days of culture and mechanical properties of the constructs appeared to be suitable for vascular applications.
Synthetic materials previously used as vascular grafts have varied, ranging from inert biomaterials such as polyethylene terephlathalate (PET, Dacron®) and expanded PTFE (Goretex©) to biodegradable synthetic polymers such as polyglycolic acid (PGA), polylactic acid (PLA) and their copolymers[14, 45-48]. While inert biomaterials do not support cell ingrowth and are not biodegradable, PGA, PLA and its copolymers have mechanical properties more suitable for orthopaedic applications due to their relatively higher glass transition temperatures and high modulus. [17] For cardiovascular applications a polymer must be elastic to be amenable to mechanical conditioning. Porosity and pore connectivity are also important to achieve successful cellular incorporation. PEUU biodegradable scaffolds meet all of these requirements and are currently being studied in several tissue engineering applications [17, 40, 49, 50]. It has previously been shown that TIPS PEUU scaffolds have a breaking strain over 214% and a tensile strength of 1.0 MPa [17, 40]. With these mechanical properties, our current approach, as opposed to a completely cellular-based approach that needs more than 8 weeks of culture,[10] would allow the TEVG to be implanted even immediately after seeding, provided that the cells are incorporated successfully.
Cell seeding constitutes a key step in those tissue-engineering approaches that incorporate cells into or onto scaffolds for culture or implantation. Desirable features for any seeding technique include: preserving cell viability, providing a uniform cell distribution, and attaining a high seeding efficiency, with reduced seeding time [38, 39]. The vast majority of seeding techniques for vascular tissue engineering involve surface seeding [51-54]. Bulk seeding is harder to accomplish (particularly in tubular constructs) and usually requires an active method of deployment [16, 39]. The vacuum seeding technique utilized in this study allowed overcoming those difficulties and also provided user independence and reproducibility which is another essential feature for large scale clinical translation.
A similar approach to ours was recently described by Shin'oka and colleagues, where a copolymer of L-lactide and e-caprolactone was used to create a TEVG that was successfully implanted in human patients as a pulmonary artery replacement [34, 55, 56]. However, in that application, most of the cells were found to be highly concentrated near the luminal surface rather than inside the polymer, prior to implantation. [56] This may be related to the manual technique utilized for the seeding.
Progenitor cells show great potential for use in tissue engineering applications and may circumvent many of the shortcomings associated with other options in cell sourcing. Progenitor cells are easier to harvest than terminally differentiated cells for vascular applications where a muscle biopsy, a bone marrow aspirate or a blood aspirate are usually preferable to a blood vessel biopsy to collect endothelial or smooth muscle cells, and usually display a rapid, almost limitless expansion capability. MDSCs are a population of long-time proliferating cells expressing hematopoietic stem cell markers that have previously shown the ability to retain their phenotype for more than 30 passages with normal karyotype and were able to differentiate into muscle, neural, and endothelial lineages both in vivo and in vitro [23, 24]. MDSCs have also been shown to have a strong self-renewal capacity and high proliferative pattern and proved to be useful in myogenic regeneration models [21, 24, 57]. Their behavior inside the PEUU scaffolds was consistent with the high proliferative features. (Fig. 3) The values for CD34 and Sca-1 expression in monolayer are similar to previous reports [23], [42, 58]. However, this is the first report to characterize the cells for their expression of CD34 and Sca-1 in a 3D environment. The observation that MDSCs maintain Sca-1 suggests that these cells maintain their stem cell phenotype throughout the seeding and culture process. CD34 is a surface glycoprotein which functions in hematopoiesis and hematopoietic cell adhesion [59, 60]. The role of CD34 expression in MDSCs function has not been fully elucidated [21, 61], although this marker is routinely used to characterize MDSC [62, 63]. We speculate here that the change in CD34 may be related to a change in the adhesion characteristics in the 3D environment. Furthermore, in another type of muscle stem cell, it has been shown that CD34 varies with the activation state of the cell [64]. The scarce expression of MHC noted at 7 days here suggests that cells are not forming myotubes, as shown in the positive controls, indicating that spontaneous differentiation to myogenic lineage is not occurring. (Fig. 5) Maintaining the stem cell phenotype during culture is desired in order to profit from the compelling regenerative capabilities of the cells when exposed to the actual vascular environment upon implantation. The performance of MDSCs within the PEUU scaffolds described in this study adds upon their previously reported potential in cell transplantation, making them an attractive source for vascular tissue engineering. It is clear that for future clinical applications a faster isolation method will be required instead of the current pre-plating technique. The potential that MDSCs have shown in different preclinical applications [65, 66] has motivated investigation of new isolation techniques, including direct cell sorting from a muscle biopsy using CD34 and Sca-1 as the cell surface antigen. [58, 61, 67]. More recently, MDSC-like cells have been isolated from human muscle biopsy using antibodies for CD56, CD34 and CD144 [68]. These studies illustrate the feasibility of prospectively isolating stem cells via cell sorting from a skeletal muscle biopsy.
It is widely accepted that a successful TEVG will not likely be constructed in static culture [8, 15] due to the decreased nutrient transfer inside the walls of the scaffold compared to dynamic culture. Indeed, this possibly explains the cell migration toward the edges observed in the controls of the current study. (Fig. 3) Several studies have utilized perfusion bioreactors to overcome this problem and to provide the TEVG with a more realistic mechanical environment, which is also thought to be important to drive the cells into the desired phenotypic lineage.[16, 20, 69, 70] We have demonstrated here that spinner flask culture has the ability to increase nutrient transfer inside a 2-cm long TEVG while maintaining the simplicity of a standard culture method, making it more attractive for clinical applications where the set-up of a complex bioreactor might represent a limiting factor.
The mechanical properties of the tubular scaffolds were demonstrated to be similar to those of native arteries. The reported burst pressure for human internal mammary arteries is 4225±1368 mmHg and the suture retention force is 1.96±1.16 N. [11], which are comparable with the values we obtained for the scaffolds.
Although MDSCs have previously been shown to differentiate into endothelial cells in vivo, one limitation of this study is the lack of a functional endothelial layer to prevent acute thrombosis upon implantation. However, anticoagulation during the initial period of the engraftment might be enough to allow tissue remodeling and complete endothelialization. Ongoing studies to test that hypothesis in vivo will help to determine whether seeding an endothelial layer in vitro is required. Another limitation of the study is the ability of these experiments to test the clinical feasibility of the spinner flask due to the length of the constructs. Longer scaffolds might require a modification of the culture system to achieve the same nutrient transfer observed in this work.
Conclusion
We describe here the in-vitro fabrication of a TEVG in which MDSCs could be effectively incorporated into biodegradable tubular scaffolds where they proliferated and produced extracellular matrix while maintaining their stem cell phenotype in spinner flask culture for 7 days. The original and successful integration of scaffold materials, stem cells, bulk seeding and dynamic culture in this approach may lead to a rapidly available TEVG, which is essential for clinical use.
Acknowledgements
The authors would like to acknowledge funding from NIH BRP #R01 HL069368 (to WRW and DAV) and AHA Postdoctoral Fellowship 0525585U (to AN). The authors would also like to thank Robert Toth, Jennifer Debbar, and Joy M. Cumer for their technical assistance, and Timothy M. Maul, Scott J. Vanepps, Douglas W. Chew and John Stankus for their scientific input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heart Disease and Stroke Statistics . 2004 Update. American Heart Association; Dallas, TX: 2004. 2004. [Google Scholar]
- 2.Fast Stats: Inpatient Surgery. [Web Site] 2003 November 14 [cited 2003 November 22]; Available from: http://www.cdc.gov/nchs/fastats/insurg.htm.
- 3.Grigioni M, Daniele C, D'Avenio G, Barbaro V. Biomechanics and Hemodynamics of Grafting. In: Tura A, editor. Vascular Grafts: Experiment and Modeling. WIT Press; Boston: 2003. pp. 41–82. [Google Scholar]
- 4.Davids L, Dower T, Zilla P. The Lack of Healing in Conventional Vascular Grafts. In: Zilla P, Greisler HP, editors. Tissue Engineering of Vascular Prosthetic Grafts. R.G. Landes; Austin: 1999. pp. 3–45. [Google Scholar]
- 5.Williams SK, Rose DG, Jarrell BE. Microvascular endothelial cell sodding of ePTFE vascular grafts: improved patency and stability of the cellular lining. Journal of Biomedical Materials Research. 1994;28(2):203–12. doi: 10.1002/jbm.820280210. [DOI] [PubMed] [Google Scholar]
- 6.Weintraub WS, Jones EL, Craver JM, Guyton RA. Frequency of repeat coronary bypass or coronary angioplasty after coronary artery bypass surgery using saphenous venous grafts. The American Journal of Cardiology. 1994;73(2):103–12. doi: 10.1016/0002-9149(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 7.Konner K. Primary vascular access in diabetic patients: an audit. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association - European Renal Association. 2000;15(9):1317–25. doi: 10.1093/ndt/15.9.1317. [DOI] [PubMed] [Google Scholar]
- 8.Nerem RM, Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3:225–43. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- 9.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 10.L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue engineered human blood vessel. The FASEB Journal. 1998;12(1):47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 11.L'Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12(3):361–5. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho SW, Lim SH, Kim IK, Hong YS, Kim SS, Yoo KJ, et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241(3):506–15. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, et al. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40(1):146–53. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Niklason LE, Abbott W, Gao J, Klagges B, Hirschi KK, Ulubayram K, et al. Morphologic and mechanical characteristics of engineered bovine arteries. Journal of Vascular Surgery. 2001;33(3):628–38. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 15.Vorp DA, Maul TM, Nieponice A. Molecular aspects of vascular tissue engineering. Frontiers in Bioscience. 2005;10:768–789. doi: 10.2741/1571. [DOI] [PubMed] [Google Scholar]
- 16.Nieponice A, Maul T, Soletti L, Vorp D. Vascular Tissue Engineering. Encyclopedia of Biomaterials and Biomedical Engineering: Taylor and Francis. 2006:1–14. [Google Scholar]
- 17.Guan J, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. Journal of Biomedical Materials Research. 2002;61(3):493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 18.Niklason LE, Langer RS. Advances in tissue engineering of blood vessels and other tissues. Transplant Immunology. 1997;5(4):303–6. doi: 10.1016/s0966-3274(97)80013-5. [DOI] [PubMed] [Google Scholar]
- 19.Nieponice A, Maul T, Cumer J, Soletti L, Vorp D. Mechanical Stimulation Induces Morphological and Phenotypic Changes in Bone Marrow-Derived Progenitor Cells Within a Three-Dimensional Fibrin Matrix. Journal of Biomedical Materials Research. 2007;81(3):523–30. doi: 10.1002/jbm.a.31041. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton DW, Maul TM, Vorp DA. Characterization of the Response of Bone Marrow Derived Progenitor Cells to Cyclic Strain: Implications for Vascular Tissue Engineering Applications. Tissue Engineering. 2004;10(34):361–70. doi: 10.1089/107632704323061726. [DOI] [PubMed] [Google Scholar]
- 21.Deasy BM, Jankowski RJ, Huard J. Muscle-derived stem cells: characterization and potential for cell-mediated therapy. Blood Cells Mol Dis. 2001;27(5):924–33. doi: 10.1006/bcmd.2001.0463. [DOI] [PubMed] [Google Scholar]
- 22.Lee JY, Qu Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. The Journal of Cell Biology. 2000;150(5):1085–100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. The Journal of Cell Biology. 2002;157(5):851–64. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deasy BM, Li Y, Huard J. Tissue engineering with muscle-derived stem cells. Curr Opin Biotechnol. 2004;15(5):419–23. doi: 10.1016/j.copbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC, et al. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332(2):370–9. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 26.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Experimental Cell Research. 2001;268(2):189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 27.Bonanno E, Ercoli L, Missori P, Rocchi G, Spagnoli LG. Homogeneous stromal cell population from normal human adult bone marrow expressing alpha-smooth muscle actin filaments. Laboratory Investigation. 1994;71(2):308–15. [PubMed] [Google Scholar]
- 28.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. Journal of Cellular Physiology. 1999;181(1):67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. Journal of Cellular Biochemistry. 1997;64(2):295–312. [PubMed] [Google Scholar]
- 30.Matsumura G, Hibino N, Ikada Y, Kurosawa H, Shin'oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24(13):2303–8. doi: 10.1016/s0142-9612(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 31.Rotter N, Haisch A, Bucheler M. Cartilage and bone tissue engineering for reconstructive head and neck surgery. Eur Arch Otorhinolaryngol. 2005;262(7):539–45. doi: 10.1007/s00405-004-0866-1. [DOI] [PubMed] [Google Scholar]
- 32.Subira M, Sureda A, Martino R, Garcia J, Altes A, Canals C, et al. Autologous stem cell transplantation for high-risk Hodgkin's disease: improvement over time and impact of conditioning regimen. Haematologica. 2000;85(2):167–72. [PubMed] [Google Scholar]
- 33.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108(18):2212–8. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 34.Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. New England Journal of Medicine. 2001;344(7):532–3. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 35.Burg KJ, Holder WD,, Jr., Culberson CR, Beiler RJ, Greene KG, Loebsack AB, et al. Comparative study of seeding methods for three-dimensional polymeric scaffolds. J Biomed Mater Res. 2000;51(4):642–9. doi: 10.1002/1097-4636(20000915)51:4<642::aid-jbm12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.Qi X, Liu JG, Chang Y, Xu XX. Comparative study on seeding methods of human bone marrow stromal cells in bone tissue engineering. Chin Med J. 2004;117(4):576–80. [PubMed] [Google Scholar]
- 37.Matsumura G, Miyagawa-Tomita S, Shin-oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108(14):1729–34. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 38.Conte M, Birinyi L. Endothelial Cell Seeding. In: Cohen J, editor. Vascular Surgery 2000. Research Strategies for the New Millenium; Landis: 2000. pp. 46–52. [Google Scholar]
- 39.Soletti LNA, Guan J, Stankus J, Wagner W, Vorp DA. A novel seeding device for tissue engineered tubular structures. Biomaterials. 2006;27(28):4863–70. doi: 10.1016/j.biomaterials.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 40.Guan J, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26(18):3961–71. doi: 10.1016/j.biomaterials.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciapetti G, Cenni E, Pratelli L, Pizzoferrato A. In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials. 1993;14(5):359–64. doi: 10.1016/0142-9612(93)90055-7. [DOI] [PubMed] [Google Scholar]
- 42.Deasy BM, Gharaibeh BM, Pollett JB, Jones MM, Lucas MA, Kanda Y, et al. Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell. 2005;16(7):3323–33. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stankus JJ, Soletti L, Fujimoto K, Hong Y, Vorp DA, Wagner WR. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials. 2007;28(17):2738–46. doi: 10.1016/j.biomaterials.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vorp DA, Schiro BJ, Ehrlich MP, Juvonen TS, Ergin MA, Griffith BP. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann Thorac Surg. 2003;75(4):1210–4. doi: 10.1016/s0003-4975(02)04711-2. [DOI] [PubMed] [Google Scholar]
- 45.Bottaro DP, Liebmann-Vinson A, Heidaran MA. Molecular signaling in bioengineered tissue microenvironments. Annals of the New York Academy of Sciences. 2002;961:143–53. doi: 10.1111/j.1749-6632.2002.tb03068.x. [DOI] [PubMed] [Google Scholar]
- 46.Kenawy eR, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, et al. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. Journal of Controlled Release. 2002;81(1−2):57–64. doi: 10.1016/s0168-3659(02)00041-x. [DOI] [PubMed] [Google Scholar]
- 47.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3(2):232–8. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 48.Dong CM, Sun XL, Faucher KM, Apkarian RP, Chaikof EL. Synthesis and characterization of glycopolymer-polypeptide triblock copolymers. Biomacromolecules. 2004;5(1):224–31. doi: 10.1021/bm0343500. [DOI] [PubMed] [Google Scholar]
- 49.Stankus JJ, Guan J, Fujimoto K, Wagner WR. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27(5):735–44. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stankus JJ, Guan J, Wagner WR. Fabrication of Biodegradable, Elastomeric Scaffolds with Sub-Micron Morphologies. Journal of Biomedical Material Research. 2004;70(4):603–14. doi: 10.1002/jbm.a.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shirota T, He H, Yasui H, Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9(1):127–36. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- 52.Mazzucotelli JP, Roudiere JL, Bernex F, Bertrand P, Leandri J, Loisance D. A new device for endothelial cell seeding of a small-caliber vascular prosthesis. Artif Organs. 1993;17(9):787–90. doi: 10.1111/j.1525-1594.1993.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 53.Bowlin GL, Rittgers SE. Electrostatic endothelial cell transplantation within small-diameter (<6 mm) vascular prostheses: a prototype apparatus and procedure. Cell Transplant. 1997;6(6):631–7. doi: 10.1177/096368979700600614. [DOI] [PubMed] [Google Scholar]
- 54.van Wachem PB, Stronck JW, Koers-Zuideveld R, Dijk F, Wildevuur CR. Vacuum cell seeding: a new method for the fast application of an evenly distributed cell layer on porous vascular grafts. Biomaterials. 1990;11(8):602–6. doi: 10.1016/0142-9612(90)90086-6. [DOI] [PubMed] [Google Scholar]
- 55.Naito Y, Imai Y, Shin'oka T, Kashiwagi J, Aoki M, Watanabe M, et al. Successful clinical application of tissue-engineered graft for extracardiac Fontan operation. J Thorac Cardiovasc Surg. 2003;125(2):419–20. doi: 10.1067/mtc.2003.134. [DOI] [PubMed] [Google Scholar]
- 56.Shin'oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129(6):1330–8. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 57.Deasy BM, Huard J. Gene therapy and tissue engineering based on muscle-derived stem cells. Curr Opin Mol Ther. 2002;4(4):382–9. [PubMed] [Google Scholar]
- 58.Jankowski RJ, Huard J. Establishing reliable criteria for isolating myogenic cell fractions with stem cell properties and enhanced regenerative capacity. Blood Cells Mol Dis. 2004;32(1):24–33. doi: 10.1016/j.bcmd.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Healy L, May G, Gale K, Grosveld F, Greaves M, Enver T. The stem cell antigen CD34 functions as a regulator of hemopoietic cell adhesion. Proc Natl Acad Sci U S A. 1995;92(26):12240–4. doi: 10.1073/pnas.92.26.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu Y, Bock G, Wick G, Xu Q. Activation of PDGF receptor alpha in vascular smooth muscle cells by mechanical stress. The FASEB Journal. 1998;12(12):1135–42. doi: 10.1096/fasebj.12.12.1135. [DOI] [PubMed] [Google Scholar]
- 61.Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem cells. Gene Ther. 2002;9(10):642–7. doi: 10.1038/sj.gt.3301719. [DOI] [PubMed] [Google Scholar]
- 62.Arriero M, Brodsky SV, Gealekman O, Lucas PA, Goligorsky MS. Adult skeletal muscle stem cells differentiate into endothelial lineage and ameliorate renal dysfunction after acute ischemia. Am J Physiol Renal Physiol. 2004;287(4):F621–7. doi: 10.1152/ajprenal.00126.2004. [DOI] [PubMed] [Google Scholar]
- 63.Hwang JH, Yuk SH, Lee JH, Lyoo WS, Ghil SH, Lee SS, et al. Isolation of muscle derived stem cells from rat and its smooth muscle differentiation [corrected]. Mol Cells. 2004;17(1):57–61. [PubMed] [Google Scholar]
- 64.Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151(6):1221–34. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yokoyama T, Pruchnic R, Lee JY, Chuang YC, Jumon H, Yoshimura N, et al. Autologous primary muscle-derived cells transfer into the lower urinary tract. Tissue Eng. 2001;7(4):395–404. doi: 10.1089/10763270152436454. [DOI] [PubMed] [Google Scholar]
- 66.Yokoyama T, Yoshimura N, Dhir R, Qu Z, Fraser MO, Kumon H, et al. Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol. 2001;165(1):271–6. doi: 10.1097/00005392-200101000-00077. [DOI] [PubMed] [Google Scholar]
- 67.Jankowski RJ, Huard J. Myogenic cellular transplantation and regeneration: sorting through progenitor heterogeneity. Panminerva Med. 2004;46(1):81–91. [PubMed] [Google Scholar]
- 68.Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25(9):1025–34. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 69.Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol. 2005;98(6):2321–2327. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 70.Stegemann JP, Nerem RM. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Annals of Biomedical Engineering. 2003;31(4):391–402. doi: 10.1114/1.1558031. [DOI] [PubMed] [Google Scholar]


