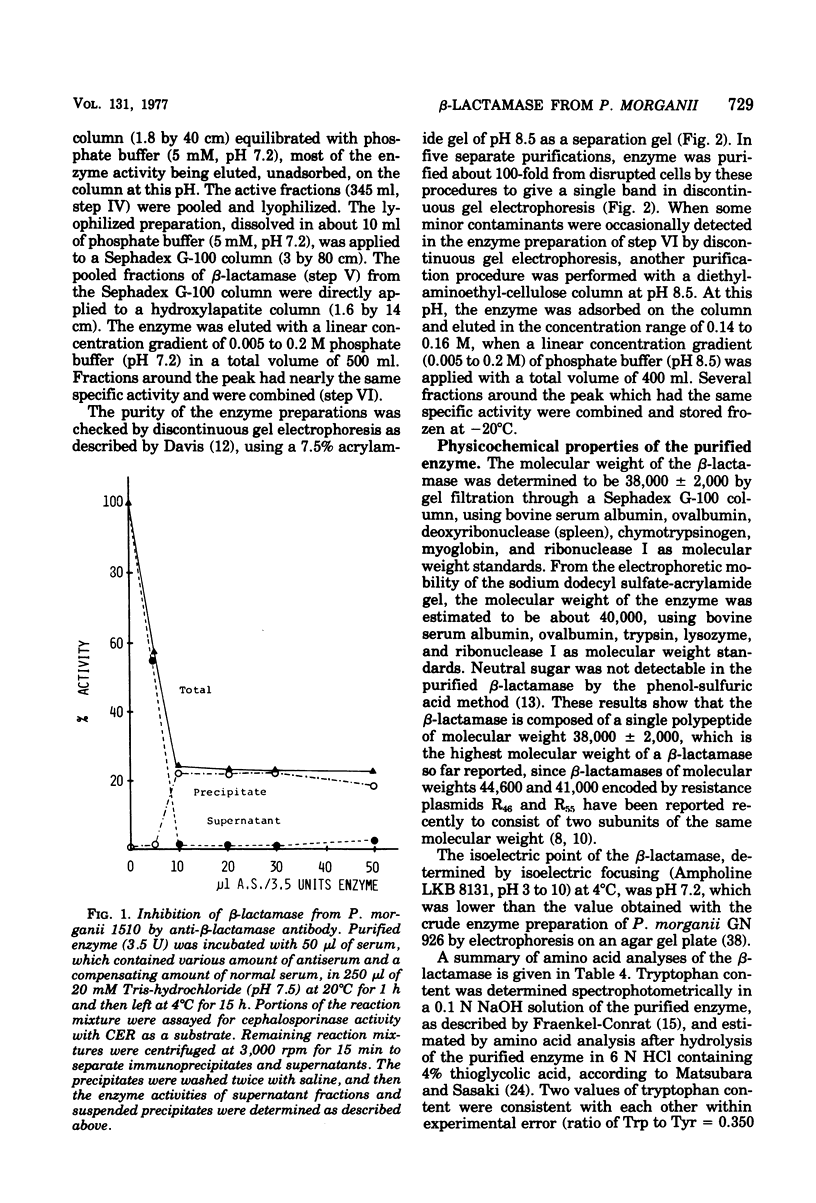
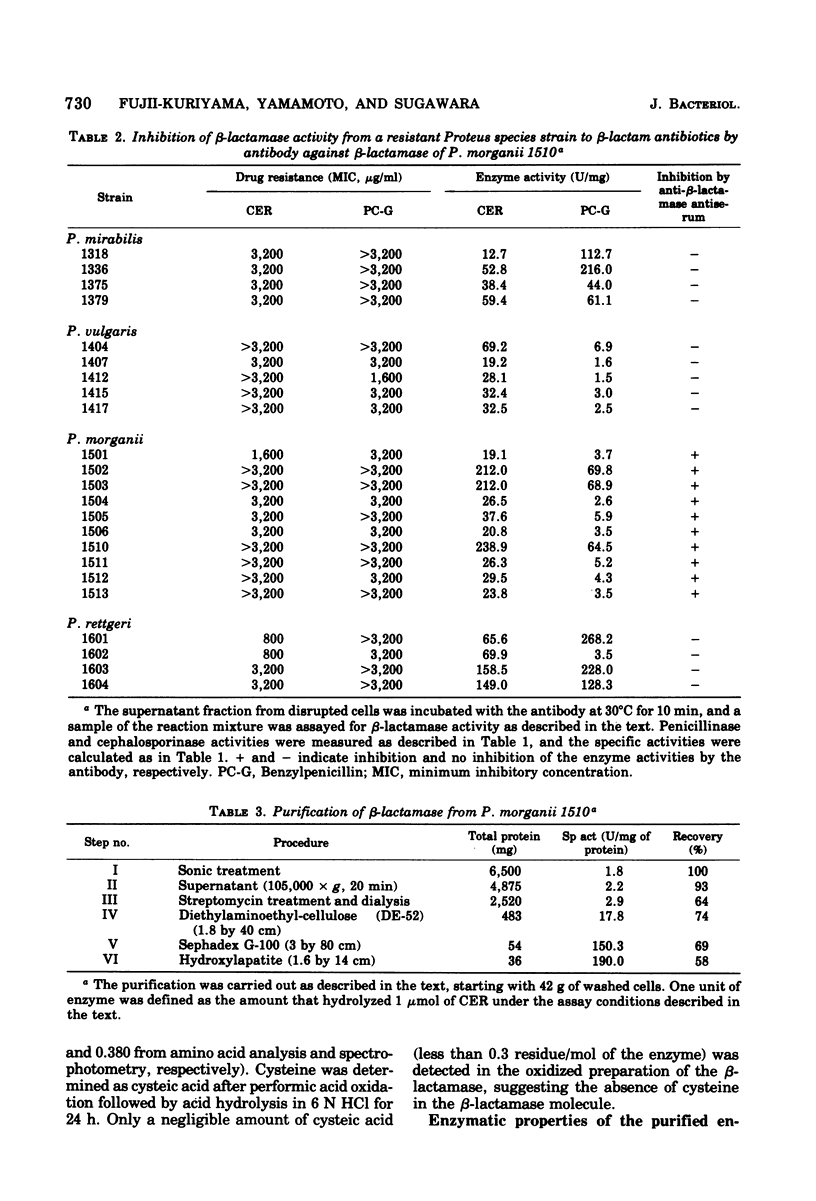
Abstract
The cephalosporin beta-lactamase was purified from a strain of Proteus morganii that showed resistance to beta-lactam antibiotics and produced the enzyme constitutively. The purified enzyme preparation gave a single protein band on polyacrylamide gel electrophoresis and consisted of a single polypeptide of molecular weight 38,000 to 40,000 from gel filtration of Sephadex G-100 and sodium dodecyl sulfate-acrylamide gel electrophoresis, its isoelectric point being pH 7.2 No cysteine residue was found in its amino acid composition. The specific activity was 190 mumol/min per mg of the purified enzyme protein for the hydrolysis of cephaloridine, the optimal pH was about 8.5 and the optimal temperature was 50 degrees C. Antibodies against the purified beta-lactamase inhibited not only the enzyme activity of the purified preparation, but also the enzyme activity of all of the other strains of P. morganii so far tested, regardless of whether the modes of their production were inducible or constitutive. None of the beta-lactamases produced by beta-lactam antibiotic-resistant strains of other species of Proteus was affected at all by the antibodies, thus showing that the purified cephalosporin beta-lactamase was of the species-specific type. The enzymological properties of the preparation have been compared with those of beta-lactamases derived from other gram-negative enteric bacteria.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayliffe G. A. Cephalosporinase and penicillinase activity of Gram-negative bacteria. J Gen Microbiol. 1965 Jul;40(1):119–126. doi: 10.1099/00221287-40-1-119. [DOI] [PubMed] [Google Scholar]
- Cole M., Elson S., Fullbrook P. D. Inhibition of the -lactamases of Escherichia coli and Klebsiella aerogenes by semi-synthetic penicillins. Biochem J. 1972 Mar;127(1):295–308. doi: 10.1042/bj1270295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dale J. W. Characterization of the -lactamase specified by the resistance factor R-1818 in E. coli K12 and other Gram-negative bacteria. Biochem J. 1971 Jul;123(4):501–505. doi: 10.1042/bj1230501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. Some relationships between R-factor and chromosomal -lactamase in Gram-negative bacteria. Biochem J. 1971 Jul;123(4):507–512. doi: 10.1042/bj1230507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. The dimeric nature of an R-factor mediated beta-lactamase. Biochem Biophys Res Commun. 1976 Feb 9;68(3):1000–1005. doi: 10.1016/0006-291x(76)91245-6. [DOI] [PubMed] [Google Scholar]
- Datta N., Richmond M. H. The purification and properties of a penicillinase whose synthesis is mediated by an R-factor in Escherichia coli. Biochem J. 1966 Jan;98(1):204–209. doi: 10.1042/bj0980204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming P. C., Goldner M., Glass D. G. Inhibition of Aerobacter cephalosporin beta-lactamase by penicillins. J Bacteriol. 1969 May;98(2):394–397. doi: 10.1128/jb.98.2.394-397.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M. INDUCIBLE PENICILLINASE IN PROTEUS MORGANI. Biochem Biophys Res Commun. 1963 Sep 10;13:43–48. doi: 10.1016/0006-291x(63)90159-1. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Smith J. T., Knox R. Interaction of cephaloridine with penicillinase-producing gram-negative bacteria. Nature. 1965 Oct 16;208(5007):235–237. doi: 10.1038/208235a0. [DOI] [PubMed] [Google Scholar]
- Hennessey T. D., Richmond M. H. The purification and some properties of a beta-lactamase (cephalosporinase) synthesized by Enterobactercloacae. Biochem J. 1968 Sep;109(3):469–473. doi: 10.1042/bj1090469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack G. W., Richmond M. H. A comparative study of eight distinct beta-lactamases synthesized by gram-negative bacteria. J Gen Microbiol. 1970 Apr;61(1):43–61. doi: 10.1099/00221287-61-1-43. [DOI] [PubMed] [Google Scholar]
- Kuriyama Y. Studies on microsomal nucleoside diphosphatase of rat hepatocytes. Its purification, intramembranous localization, and turnover. J Biol Chem. 1972 May 25;247(10):2979–2988. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindqvist R. C., Nordström K. Resistance of Escherichia coli to penicillins. VII. Purification and characterization of a penicillinase mediated by the R factor R1. J Bacteriol. 1970 Jan;101(1):232–239. doi: 10.1128/jb.101.1.232-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linström E. B., Boman H. G., Steele B. B. Resistance of Escherichia coli to penicillins. VI. Purification and characterization of the chromosomally mediated penicillinase present in ampA-containing strains. J Bacteriol. 1970 Jan;101(1):218–231. doi: 10.1128/jb.101.1.218-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Muggleton P. W. The action of cephaloridine with cloxacillin or methicillin against beta-lactamase-producing gram-negative bacteria. J Gen Microbiol. 1967 Sep;48(3):449–460. doi: 10.1099/00221287-48-3-449. [DOI] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Sawai T., Mitsuhashi S., Yamagishi S. Drug resistance of enteric bacteria. XIV. Comparison of beta-lactamases in gram-negative rod bacteria resistant to alpha-aminobenzylpenicillin. Jpn J Microbiol. 1968 Dec;12(4):423–434. doi: 10.1111/j.1348-0421.1968.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Sawai T., Takahashi K., Yamagishi S., Mitsuhashi S. Variant of penicillinase mediated by an R factor in Escherichia coli. J Bacteriol. 1970 Nov;104(2):620–629. doi: 10.1128/jb.104.2.620-629.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Yamagishi S. [Beta-lactamase, a penicillin/cephalosporin hydrolyzing enzyme (author's transl)]. Tanpakushitsu Kakusan Koso. 1975 Nov;20(13):1202–1213. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yaginuma S., Sawai T., Ono H., Yamagishi S., Mitsuhashi S. Biochemical properties of a cephalosporin beta-lactamase from Pseudomonas aeruginosa. Jpn J Microbiol. 1973 Mar;17(2):141–149. doi: 10.1111/j.1348-0421.1973.tb00718.x. [DOI] [PubMed] [Google Scholar]
- Yaginuma S., Sawai T., Yamagishi S., Mitsuhashi S. Beta-lactamase formation and resistance of Proteus morganii to various penicillins and cephalosporins. Jpn J Microbiol. 1974 Mar;18(2):113–118. doi: 10.1111/j.1348-0421.1974.tb00798.x. [DOI] [PubMed] [Google Scholar]
- Yamagishi S., O'Hara K., Sawai T., Mitsuhashi S. The purification and properties of penicillin beta-lactamases mediated by transmissible R factors in Escherichia coli. J Biochem. 1969 Jul;66(1):11–20. doi: 10.1093/oxfordjournals.jbchem.a129111. [DOI] [PubMed] [Google Scholar]




