Abstract
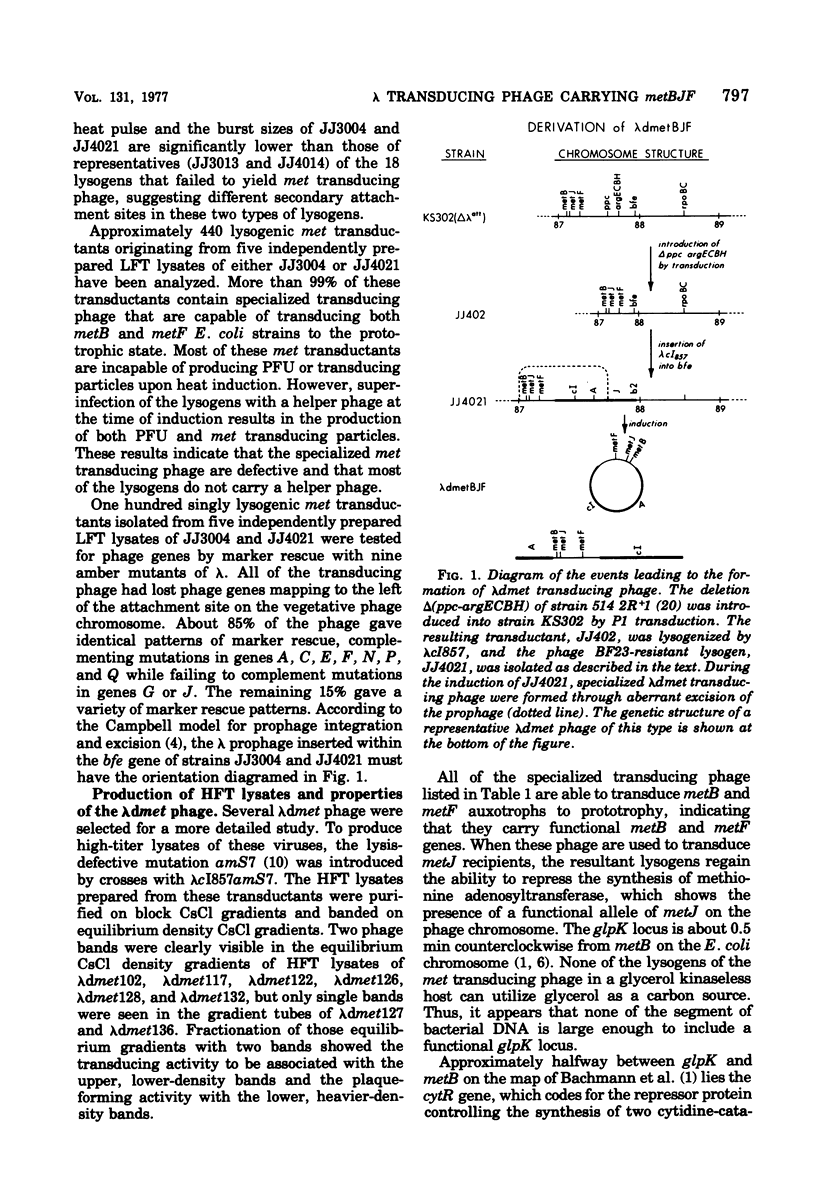
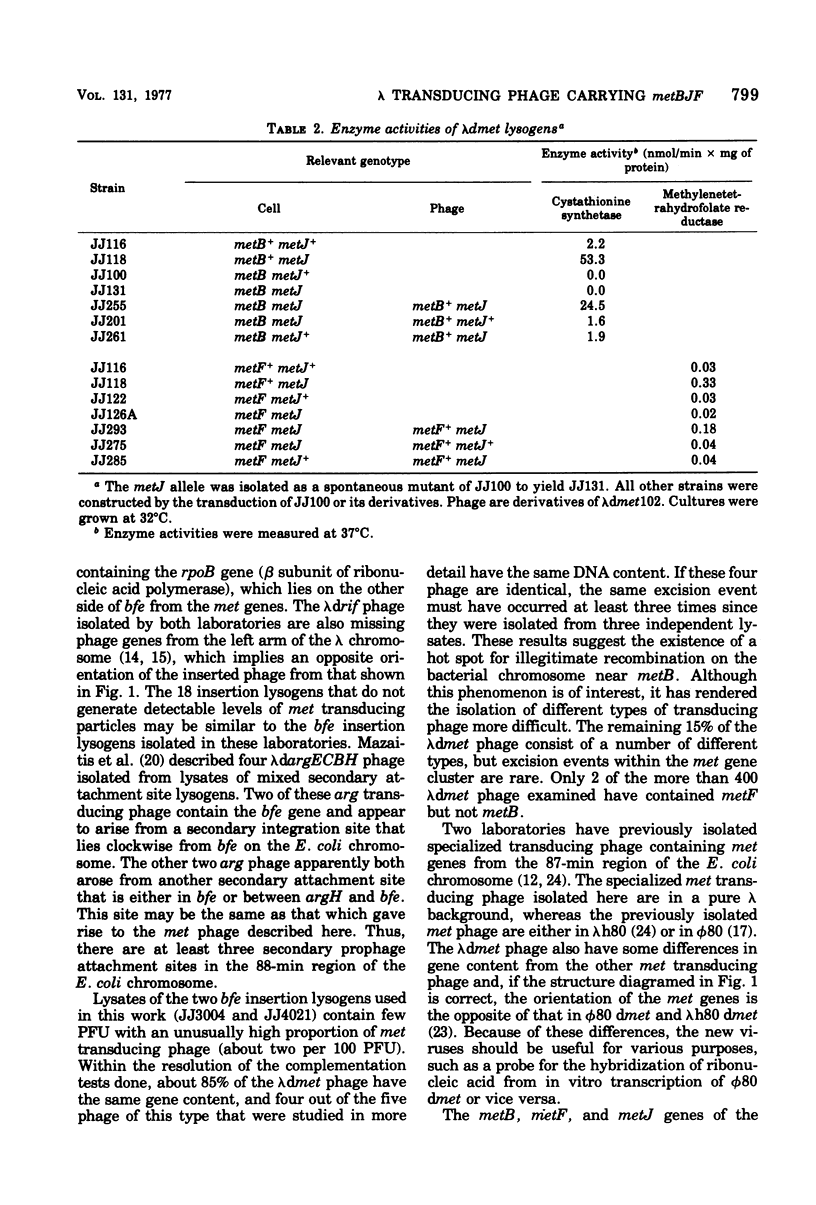
Secondary attachment site lysogens of ΔattλΔppc-argECBH strains of Escherichia coli with λcI857 integrated into the bfe gene (88 min) were isolated. Of 20 such lysogens examined, 2 produce lysates with transducing phage containing the metBJF gene cluster (87 min). Reintroduction of the ppc-argECBH chromosome segment (which lies between the bfe and met genes) into these strains virtually abolishes the production of met transducing phage. All of the phage examined have lost essential genes from the left arm of the λ chromosome. Approximately 85% of the phage appear to have the same genetic composition, containing the metBJF gene cluster, but not the closely linked gene cytR, and having lost phage genes G and J. Analytical CsCl density gradient centrifugation of five representatives of this major class of phage shows four of them to have identical densities (lighter than λ), while the fifth cannot be resolved from λ. The four apparently identical phage were isolated from three separate lysates, which suggests the existence of preferred sites for illegitimate recombination on the bacterial and phage chromosomes. Three specialized transducing phage that carry cytR in addition to metB, metJ, and metF have also been studied. Each of these viruses has a different amount of phage deoxyribonucleic acid. Two of them have less deoxyribonucleic acid than λ, whereas the third has about the same amount. The metB, metF, and cytR genes of the transducing phage have been shown to function in vivo. The phage-borne metB and metF genes are subject to metJ-mediated repression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R. S. Genetic analysis of Escherichia coli K12 mutants resistant to bacteriophage BF23 and the E-group colicins. Mol Gen Genet. 1971;113(2):154–156. doi: 10.1007/BF00333188. [DOI] [PubMed] [Google Scholar]
- Coward J. K., Chello P. L., Cashmore A. R., Parameswaran K. N., DeAngelis L. M., Bertino J. R. 5-methyl-5,6,7,8-tetrahydropteroyl oligo-gamma-L-glutamates: synthesis and kinetic studies with methionine synthetase from bovine brain. Biochemistry. 1975 Apr 8;14(7):1548–1552. doi: 10.1021/bi00678a032. [DOI] [PubMed] [Google Scholar]
- DONALDSON K. O., KERESZTESY J. C. Further evidence on the nature of prefolic A. Biochem Biophys Res Commun. 1961 Jul 26;5:289–292. doi: 10.1016/0006-291x(61)90165-6. [DOI] [PubMed] [Google Scholar]
- Flavin M., Slaughter C. Synthesis of the succinic ester of homoserine, a new intermediate in the bacterial biosynthesis of methionine. Biochemistry. 1965 Jul;4(7):1370–1375. doi: 10.1021/bi00883a022. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R., Howe M. New mutations in the S cistron of bacteriophage lambda affecting host cell lysis. Virology. 1969 May;38(1):200–202. doi: 10.1016/0042-6822(69)90148-2. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Greene R. C., Hunter J. S., Coch E. H. Properties of metK mutants of Escherichia coli K-12. J Bacteriol. 1973 Jul;115(1):57–67. doi: 10.1128/jb.115.1.57-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway C. T., Greene R. C., Su C. H. Regulation of S-adenosylmethionine synthetase in Escherichia coli. J Bacteriol. 1970 Nov;104(2):734–747. doi: 10.1128/jb.104.2.734-747.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi T., Yura T., Yamagishi H. Genetic and physical studies of lambda transducing bacteriophage carrying the beta subunit gene of the Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1975 Jun;122(3):1247–1256. doi: 10.1128/jb.122.3.1247-1256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Kirschbaum J. B., Konrad E. B. Isolation of a specialized lambda transducing bacteriophage carrying the beta subunit gene for Escherichia coli ribonucleic acid polymerase. J Bacteriol. 1973 Nov;116(2):517–526. doi: 10.1128/jb.116.2.517-526.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad B., Kirschbaum J., Austin S. Isolation and characterization of phi80 transducing bacteriophage for a ribonucleic acid polymerase gene. J Bacteriol. 1973 Nov;116(2):511–516. doi: 10.1128/jb.116.2.511-516.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. A., Smith D. A., Rowbury R. J. Regulation of methionine synthesis in Salmonella typhimurium: mutants resistant to inhibition by analogues of methionine. Genetics. 1968 Apr;58(4):473–492. doi: 10.1093/genetics/58.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaitis A. J., Palchaudhuri S., Glansdorff N., Maas W. K. Isolation and characterization of lambdadargECBH transducing phages and heteroduplex analysis of the argECBH cluster. Mol Gen Genet. 1976 Jan 16;143(2):185–196. doi: 10.1007/BF00266921. [DOI] [PubMed] [Google Scholar]
- McKenzie R. M., Gholson R. K. A simple assay for methionine adenosyltransferase using cation exchange paper and liquid scintillation spectrometry. Anal Biochem. 1973 Jun;53(2):384–391. doi: 10.1016/0003-2697(73)90084-5. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen A., Nygaard P., Hammer-Jespersen K., Fiil N. Mutants constitutive for nucleoside-catabolizing enzymes in Escherichia coli K12. Isolation, charactrization and mapping. Eur J Biochem. 1972 May 23;27(2):208–215. doi: 10.1111/j.1432-1033.1972.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Otsubo E., Lee H. J., Deonier R. C., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. VI. Mapping of F14 sequences homologous to phi 80dmetBJF and phi 80dargECBH bacteriophages. J Mol Biol. 1974 Nov 15;89(4):599–618. doi: 10.1016/0022-2836(74)90038-2. [DOI] [PubMed] [Google Scholar]
- Press R., Glansdorff N., Miner P., De Vries J., Kadner R., Maas W. K. Isolation of transducing particles of phi-80 bacteriophage that carry different regions of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1971 Apr;68(4):795–798. doi: 10.1073/pnas.68.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. II. Mutations induced by bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1973 Oct 25;80(2):297–314. doi: 10.1016/0022-2836(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Su C. H., Greene R. C. Regulation of methionine biosynthesis in Escherichia coli: mapping of the metJ locus and properties of a metJ plus-metJ minus diploid. Proc Natl Acad Sci U S A. 1971 Feb;68(2):367–371. doi: 10.1073/pnas.68.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]


