Abstract
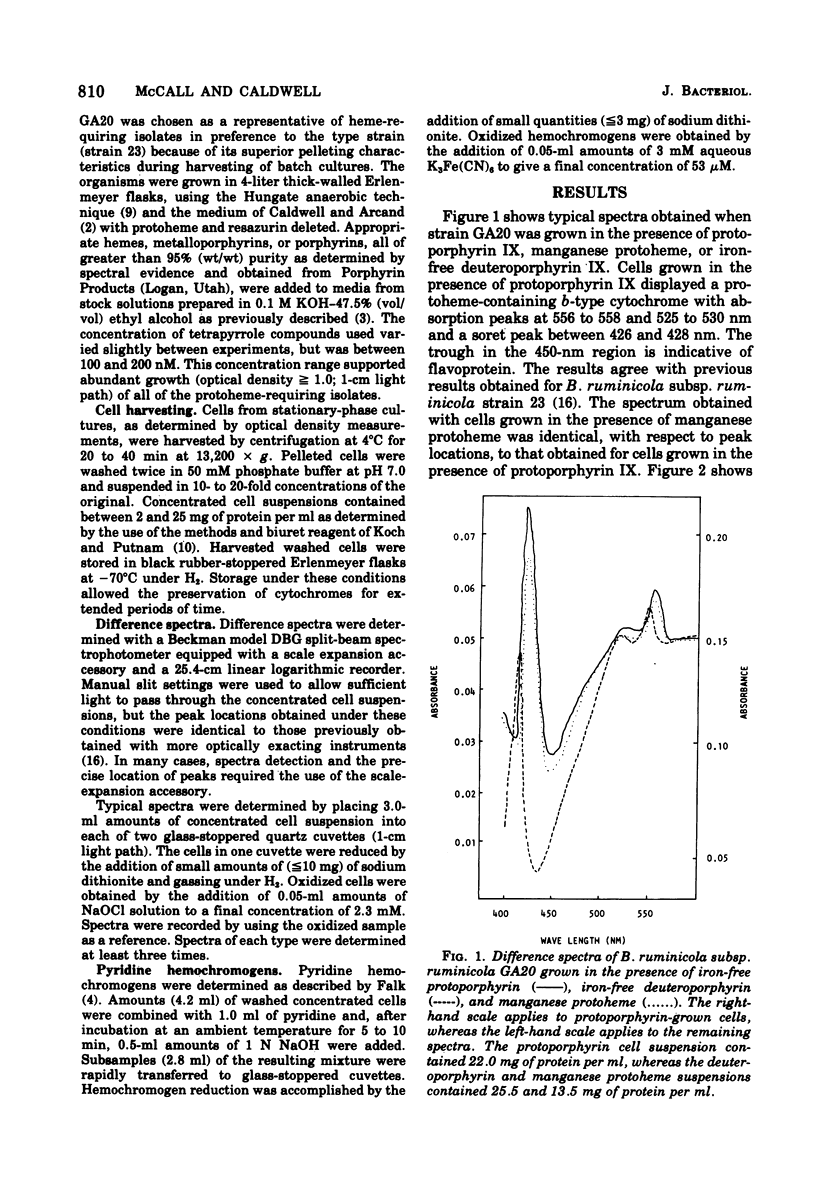
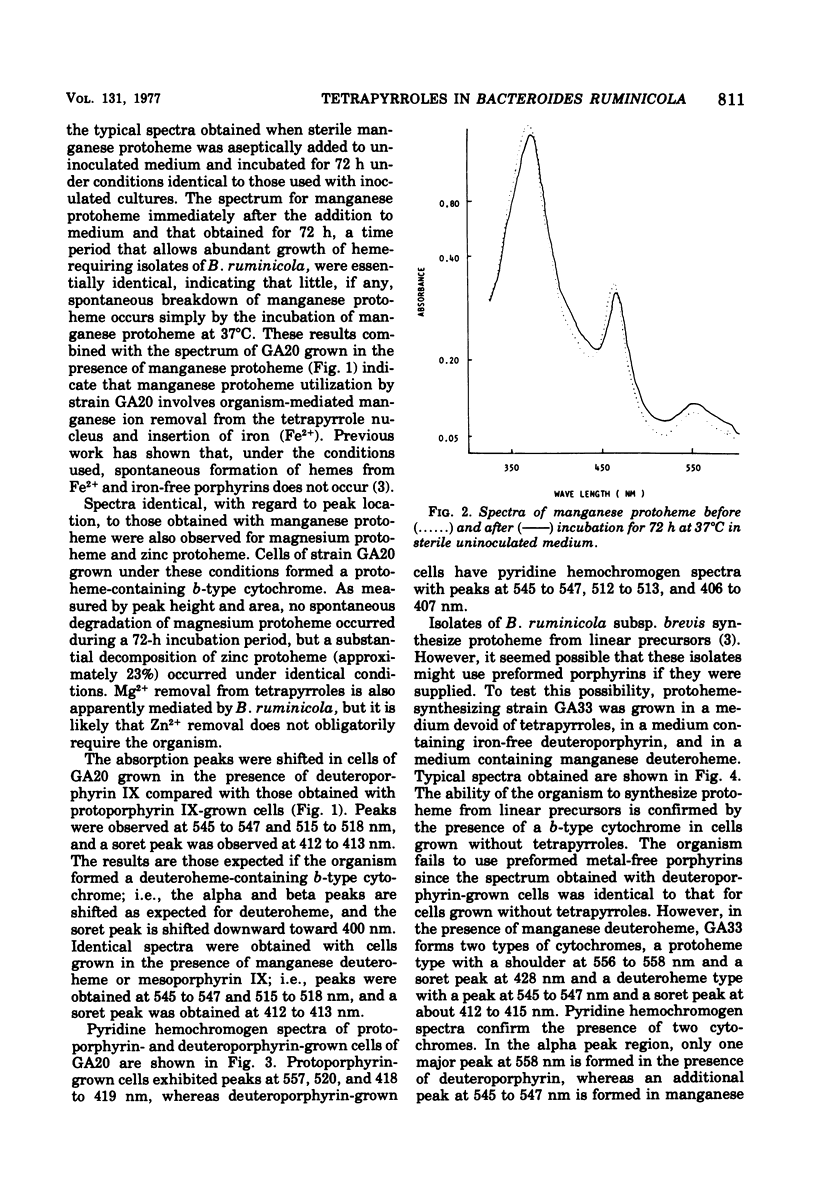
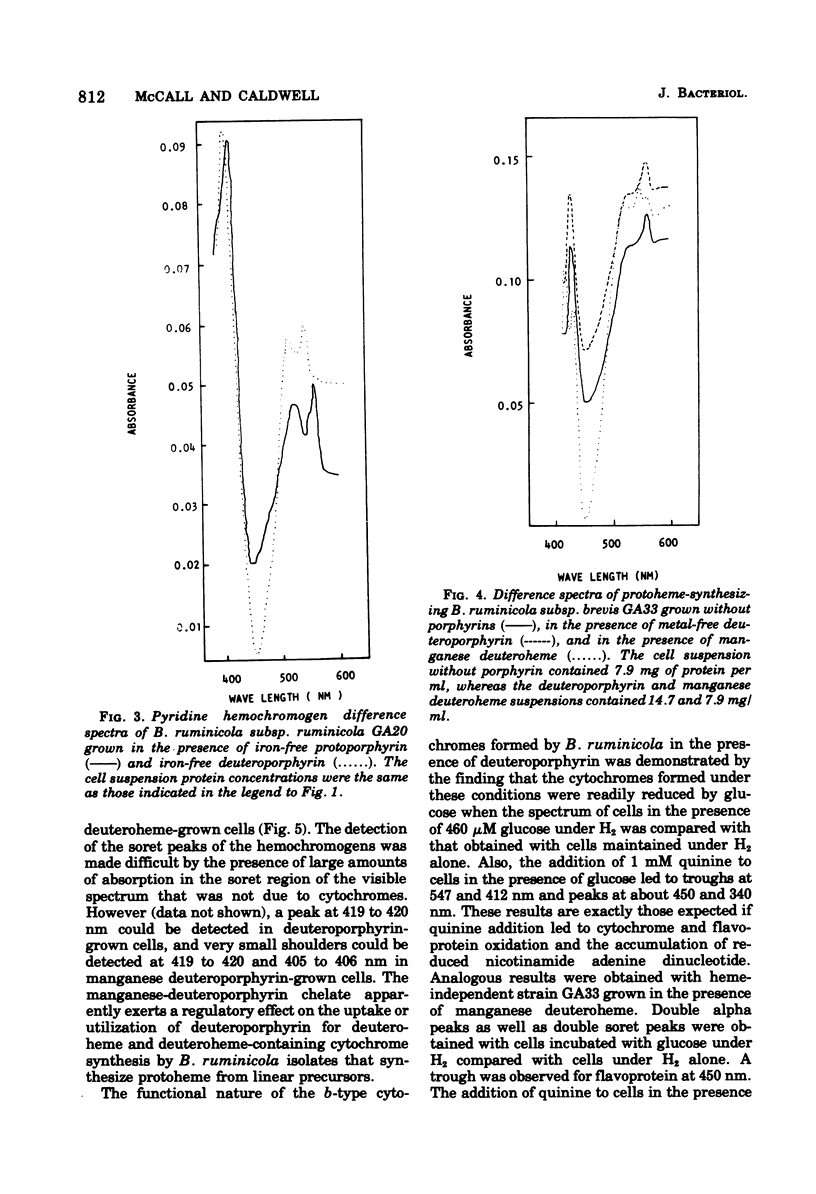
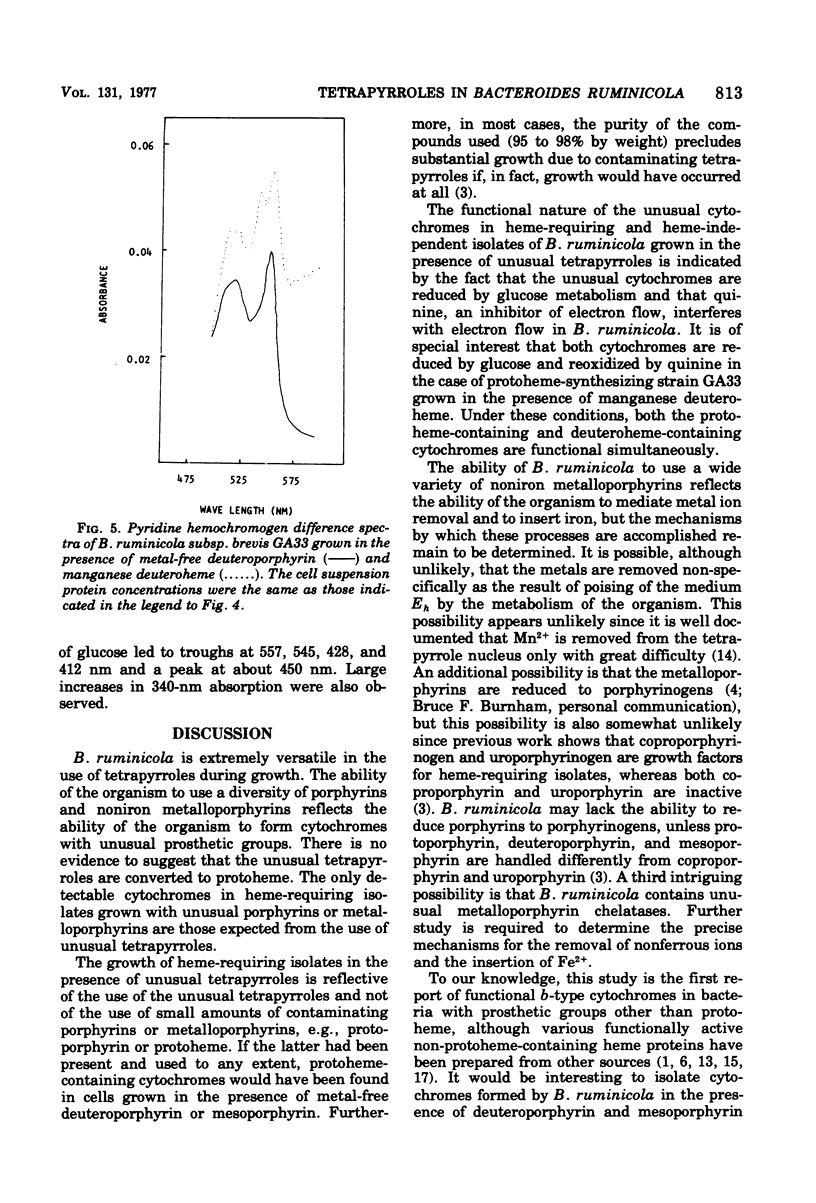
Reduced versus oxidized difference spectra of whole cells and pyridine hemochromogens of heme-requiring isolates of Bacteroides ruminicola are altered when deuteroporphyrin or mesoporphyrin replaces protoheme as a growth factor. During growth in the presence of either deuteroporphyrin or mesoporphyrin, whole cells exhibit peaks at 545 t547, 515 to 518, and 412 to 413 nm. Pyridine hemochromogen spectra confirm the presence of meso -or deuteroheme in cells grown in the presence of meso- or deuteroporphyrin. No evidence was found for the conversion of either meso- or deuteroporphyrin to protoheme. Cells grown in the presence of the manganese of magnesium chelates of protoheme form iron-containing hemes. Neither spontaneous decomposition of noniron metalloporphyrin chelates nor spontaneous formation of hemes from Fe2+ and metal-free porphyrins was detected. Protoheme-synthesizing isolates of B. ruminicola fail to use preformed metal-free porphyrins, but form both protoheme- and deuteroheme-containing cytochromes when grown in the presence of manganese deuteroheme. Versatility in tetrapyrrole utilization by B. ruminicola appears to reflect the ability of the organism to mediate the removal of nonferrous ions and to insert Fe2+ into the tetrapyrrole nucleus. The orgamism also forms functional b-type cytochromes with prosthetic groups other than protoheme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell D. R., Arcand C. Inorganic and metal-organic growth requirements of the genus Bacteroides. J Bacteriol. 1974 Oct;120(1):322–333. doi: 10.1128/jb.120.1.322-333.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., White D. C., Bryant M. P., Doetsch R. N. Specificity of the heme requirement for growth of Bacteroides ruminicola. J Bacteriol. 1965 Dec;90(6):1645–1654. doi: 10.1128/jb.90.6.1645-1654.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J Bacteriol. 1960 Aug;80:164–170. doi: 10.1128/jb.80.2.164-170.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H. THE COMBINATION OF PORPHYRINS WITH NATIVE HUMAN GLOBIN. J Biol Chem. 1964 Oct;239:3282–3287. [PubMed] [Google Scholar]
- Koch A. L., Putnam S. L. Sensitive biuret method for determination of protein in an impure system such as whole bacteria. Anal Biochem. 1971 Nov;44(1):239–245. doi: 10.1016/0003-2697(71)90366-6. [DOI] [PubMed] [Google Scholar]
- SMITH M. H., GIBSON Q. H. The preparation and some properties of myoglobin containing meso- and deutero- haem. Biochem J. 1959 Sep;73:101–106. doi: 10.1042/bj0730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C., BRYANT M. P., CALDWELL D. R. Cytochromelinked fermentation in Bacteroides ruminicola. J Bacteriol. 1962 Oct;84:822–828. doi: 10.1128/jb.84.4.822-828.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonetani T., Yamamoto H., Woodrow G. V., 3rd Studies on cobalt myoglobins and hemoglobins. I. Preparation and optical properties of myoglobins and hemoglobins containing cobalt proto-, meso-, and deuteroporphyrins and thermodynamic characterization of their reversible oxygenation. J Biol Chem. 1974 Feb 10;249(3):682–690. [PubMed] [Google Scholar]



