Abstract
Genetic studies in chickens and receptor interference experiments have indicated that avian leukosis virus (ALV)-E may utilize a cellular receptor related to the receptor for ALV-B and ALV-D. Recently, we cloned CAR1, a tumor necrosis factor receptor (TNFR)-related protein, that serves as a cellular receptor for ALV-B and ALV-D. To determine whether the cellular receptor for ALV-E is a CAR1-like protein, a cDNA library was made from turkey embryo fibroblasts (TEFs), which are susceptible to ALV-E infection, but not to infection by ALV-B and ALV-D. The cDNA library was screened with a radioactively labeled CAR1 cDNA probe, and clones that hybridized with the probe were isolated. A 2.3-kb cDNA clone was identified that conferred susceptibility to ALV-E infection, but not to ALV-B infection, when expressed in transfected human 293 cells. The functional cDNA clone is predicted to encode a 368 amino acid protein with significant amino acid similarity to CAR1. Like CAR1, the TEF protein is predicted to have two extracellular TNFR-like cysteine-rich domains and a putative death domain similar to those of TNFR I and Fas. Flow cytometric analysis and immunoprecipitation experiments demonstrated specific binding between the TEF CAR1-related protein and an immunoadhesin composed of the surface (SU) envelope protein of subgroup E (RAV-0) virus fused to the constant region of a rabbit immunoglobulin. These two activities of the TEF CAR1-related protein, specific binding to ALV-E SU and permitting entry only of ALV-E, have unambiguously identified this protein as a cellular receptor specific for subgroup E ALV.
Retroviral infection is initiated through interactions between the viral envelope protein (Env) and specific receptors present on the surface of the host cell. The viral surface (SU) Env protein directly binds to the viral receptor, and subsequently conformational changes in Env that expose fusion peptide regions of the transmembrane (TM) Env protein are thought to drive fusion of the viral and cellular membranes (1, 2). We are using avian leukosis virus (ALV) receptor interactions as a model system to understand how retroviruses enter their host cells. There are six well characterized chicken subgroups of ALV (A–E and J) which are defined on the basis of host range, antibody neutralization, and receptor interference studies (1).
Subgroups B, D, and E of ALV are predicted to utilize the same or related receptors in susceptible chicken cells. One line of evidence that supports this hypothesis comes from receptor interference studies. Cells preinfected with either ALV-B or ALV-D are resistant to superinfection by subgroup B, D, and E viruses, presumably because of newly synthesized viral Env binding to the receptor, thus preventing subsequent rounds of viral entry (1). However, cells preinfected with ALV-E are resistant only to superinfection by subgroup E viruses. The reason for the nonreciprocal receptor interference pattern exhibited by these viruses remains to be determined.
Further evidence that these viruses use the same or related receptors comes from genetic studies in chickens which indicated that several alleles of a single locus, tv-b, encode receptors for subgroups B, D, and E viruses (1, 3, 4). The tv-bs1 allele was proposed to confer susceptibility to infection by all three viral subgroups. The tv-bs3 allele was proposed to allow infection only by subgroups B and D ALV, and the tv-br allele does not permit entry by any of these viruses (1).
Previously we identified CAR1, a receptor specific for ALV-B and ALV-D, but not for ALV-E, from chickens homozygous for the tv-bs3 allele (5). CAR1 is a tumor necrosis factor receptor (TNFR)-related protein with two extracellular cysteine-rich domains (CRDs), which are characteristic of this family, and it contains a putative death domain like those found in TNFR-I and Fas (5). The presence of a death domain in this receptor probably explains, at least in part, why subgroup B and D ALV infection causes a transient cytopathic effect in chicken embryo fibroblasts (CEFs) as a result of apoptosis induced by the binding of the viral SU protein to CAR1 (5). In contrast, infections by subgroup E viruses have been observed to be noncytopathic (6–8).
We were interested in identifying the receptor for ALV-E for two principal reasons. First, identifying and characterizing this receptor would allow a comparison between the mechanisms of infection used by subgroup E virus with those used by subgroup B and D viruses. Second, it would allow an investigation of the precise reason why some ALV subgroups, but not others, lead to cytopathic infections of CEFs. To obtain a subgroup E-specific viral receptor, we used turkey cells which, in contrast to chickens, are resistant to ALV-B and ALV-D, but are susceptible to ALV-E infection. We now report that a turkey protein that is related to CAR1 is a cellular receptor specific for subgroup E ALV.
MATERIALS AND METHODS
Cells and Viruses.
Primary turkey embryo fibroblasts (TEFs) were obtained from Suzanne Ortiz (National Institutes of Health), and line 15B1 primary CEFs were a generous gift of Connie Cepko (Harvard Medical School). Human 293 cells, TEFs, and CEFs were grown as previously described (9–11). RCASBP(B)/AP DNA was a gift from Mark Federspiel (Mayo Foundation), and RCASBP(E)/AP was a gift from Connie Cepko. To generate these viral stocks, proviral DNA was transfected into CEFs by the calcium phosphate method as described previously (9).
cDNA Cloning and Sequencing.
Total RNA was isolated from TEFs as described previously (5). Approximately 5 μg of polyadenylated mRNA, isolated from 125 μg of total RNA by using the RNA Isolation Kit (Stratagene), was reverse-transcribed to generate cDNA using a commercially available kit (ZAP cDNA synthesis kit; Stratagene) and introduced into the λZAP Express vector (Stratagene). The cDNA library of approximately 200,000 clones was screened as described previously (5), with the exception that the radioactively labeled probe was a 2.2-kb SpeI/NotI fragment that contained the entire CAR1 cDNA clone (5). Filter hybridization was performed as described before (5), with the exception that the hybridization temperature used was 60°C and the filters were washed in 2× SSC/0.1% SDS, also at 60°C. Two rounds of screening led to the isolation of individual bacteriophage clones. By using a standard phagemid excision protocol (Stratagene), the plasmid pBKTEF24 was then isolated, and this cDNA clone was subsequently sequenced by the chain termination method (12).
Transfections and Alkaline Phosphatase (AP) Assays.
Human 293 cells, plated at approximately 20% confluency on 100-mm tissue culture plates, were transfected with 10 μg of plasmid pBK7.6–2 DNA encoding CAR1 (5), 10 μg of plasmid pBKTEF24, or no DNA (mock). Cells were split into six-well tissue culture dishes 72 hr after transfection and incubated with 2 ml of medium containing 50 μl of RCASBP(B)/AP or RCASBP(E)/AP virus. Three days after viral challenge, cells were stained for AP activity by using a protocol adapted from those described previously (13, 14). Briefly, cells were washed once with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 15 min at room temperature. Cells were then washed three times with PBS, and the endogenous AP was inactivated by incubating at 65°C for 30 min. Subsequently, cells were washed three times with AP detection buffer (100 mM Tris⋅HCl, pH 9.5/100 mM NaCl/50 mM MgCl2) and then incubated in 330 μg/ml nitroblue tetrazolium chloride (NBT) and 165 μg/ml 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt (BCIP) (GIBCO/BRL) in AP detection buffer at room temperature in the dark for 15 min. The reaction was terminated with PBS supplemented with 20 mM EDTA, and the numbers of purple foci of infected cells were counted.
SU-Immunoadhesin Construction, Immunoprecipitations, and Immunoblotting.
Plasmid pKZ452, which encodes the SUB-rIgG immunoadhesin comprising the subgroup B RAV-2 SU protein fused to the Fc portion of a rabbit immunoglobulin, was described previously (5). Plasmid pCIE-rIgG, encoding an immunoadhesin containing a subgroup E-specific SU protein, was generated by a triple ligation of the following DNA fragments: (i) KpnI/NotI-cut plasmid pCI (Promega) vector DNA, (ii) a 1.2-kb KpnI/HindIII fragment of RCASBP(E)/AP proviral DNA encoding the majority of the RAV(0) SU, including the subgroup E determining region, and (iii) a 0.84-kb HindIII/NotI fragment encoding 26 C-terminal residues of the subgroup A (SR-A) SU protein (missing the terminal lysine and arginine) fused to the Fc portion of a rabbit immunoglobulin (11). The SUB-rIgG and SUE-rIgG proteins were produced in the extracellular supernatants of transiently transfected 293 cells as described previously (5, 11).
Human 293 cells were transfected with plasmid pBK7.6–2, with plasmid pBKTEF24, or no DNA (mock), and cell lysates were prepared as described previously (5). Immunoprecipitations were performed by pre-binding 1 ml of extracellular supernatants containing immunoadhesin to 25 μl of staphylococcal protein A-Sepharose (Sigma) and 75 μl of Sepharose CL-4B (Sigma) by rocking at 4°C for 1 hr. The immunoadhesin-bound protein A-Sepharose beads were then washed three times in ice-cold Nonidet P-40 lysis buffer and incubated with approximately 800 μg of protein lysate at 4°C for 1 hr. The beads were then collected by centrifugation and washed three times with ice-cold Nonidet P-40 lysis buffer, and the proteins were eluted from the pelleted beads by boiling in sample buffer. All samples were subjected to electrophoresis on an SDS/12% polyacrylamide gel under reducing conditions. The proteins were transferred to a nitrocellulose filter and probed first with the polyclonal rabbit IQS579 antibody and then with horseradish peroxidase (HRP)-conjugated donkey antibody specific for rabbit immunoglobulins (Amersham). Bound antibodies were detected by enhanced chemiluminescence (Amersham). The IQS579 antibody was generated by Research Genetics, using a keyhole limpet hemocyanin-coupled peptide of residues 57–79 of CAR1 (5).
Flow Cytometric Analysis of Transfected Cells.
Human 293 cells were transfected as described above. Approximately 60 hr after transfection, cells were prepared for flow cytometry as described (11). Cells were bound to the immunoadhesin by incubation with 1 ml of extracellular supernatants containing approximately equal amounts of either SUB-rIgG or SUE-rIgG as assessed by immunoblotting with an HRP-conjugated anti-rabbit antibody as a probe (data not shown). Samples of 5,000 cells were then analyzed on a Coulter Epics XL Flow Cytometer.
RESULTS
Isolation of a cDNA Clone That Permits Infection by ALV-E.
To determine whether subgroup E ALV utilizes a CAR1-like receptor to infect turkey cells, Southern and Northern blot analyses were first used to demonstrate that TEFs contain a single gene highly related to CAR1 and express a 2.3-kb CAR1-related mRNA transcript (data not shown). Subsequently, a cDNA library was prepared from TEFs, and the library was screened with a CAR1 cDNA probe (as described in Materials and Methods). Individual cDNA clones that hybridized with this probe were identified, and those greater than 2 kb in size were isolated in a mammalian expression vector and then tested for their ability to confer susceptibility to ALV-E infection when expressed in transfected human 293 cells, normally not permissive for subgroup E virus infection.
After transfection, cells were challenged with an ALV-based retroviral vector containing Env proteins derived either from a subgroup E virus, RCASBP(E)/AP (14), or from a subgroup B virus, RCASBP(B)/AP. Both of these viruses contain a gene encoding heat stable AP in place of src (14). After heat inactivation of endogenous AP, this heat-stable AP will cleave a chromogenic substrate that specifically stains infected cells purple. Three days after the viral challenge, cells were stained by this procedure and the number of foci of AP-positive cells was counted.
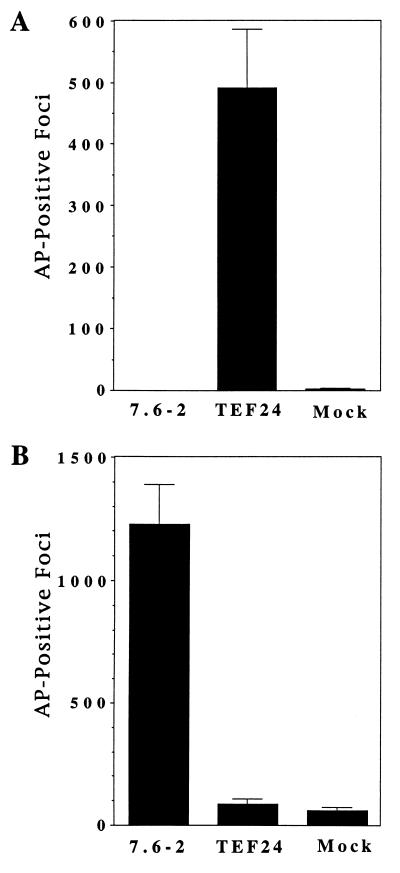
Human 293 cells transfected with one of the TEF clones, pBKTEF24, were infected by the subgroup E virus, in contrast to cells transfected with plasmid pBK7.6–2 expressing CAR1 or mock transfected cells (Fig. 1A). Similar results were obtained with several independent clones derived from two TEF cDNA libraries (data not shown). Conversely, cells transfected with plasmid pBK7.6–2 expressing CAR1 were much more susceptible to infection by the subgroup B virus than were pBKTEF24-transfected cells or mock-transfected cells (Fig. 1B). Human 293 cells appear to have a low level of susceptibility to ALV-B infection, because they were also infected by another subgroup B virus containing the hygromycin B phosphotransferase gene (J.B. and J.A.T.Y., unpublished results). Taken together, these data demonstrated that the pBKTEF24 cDNA clone conferred susceptibility to infection specifically by ALV-E when expressed in transfected human 293 cells.
Figure 1.
The pBKTEF24 plasmid specifically confers susceptibility to ALV-E infection when transfected in human 293 cells. The 293 cells were transiently transfected with plasmid pBKTEF24, plasmid pBK7.6–2 encoding CAR1 (5), or no DNA (mock). The transfected cells were challenged with either a subgroup E virus, RCASBP(E)/AP (13) (A), or a subgroup B virus, RCASBP(B)/AP (B). Three days after viral challenge, cells were stained to identify those that expressed the virally encoded heat-stable AP, and the stained foci were counted. The data shown represent the average number of foci obtained in three independent experiments.
The pBKTEF24 cDNA Clone Encodes a CAR1-Like Protein.
The pBKTEF24 cDNA clone was sequenced, and an open reading frame was identified encoding a 368 amino acid type I membrane protein. This protein is predicted to contain a 21 amino acid signal peptide, a 135 amino acid extracellular region, a 24 amino acid single transmembrane region, and a 188 amino acid cytoplasmic tail (Fig. 2). Comparison of the amino acid sequence of this protein with that of CAR1 revealed that these two proteins are highly related (Fig. 3). Like CAR1, the TEF protein is predicted to have two extracellular TNFR-like CRDs and a putative death domain similar to the death domains of TNFR-I and Fas (Fig. 3).
Figure 2.
The pBKTEF24 clone encodes a type I transmembrane protein. The complete DNA sequence of the pBKTEF24 cDNA clone is shown with the predicted amino acid sequence of the protein product. The putative transmembrane domain is underlined and the three potential N-linked glycosylation sites (N-X-S/T) are boxed. The predicted leader peptidase cleavage site (15) is marked with an arrow.
Figure 3.
The TEF protein is highly related to CAR1. The amino acid sequence of the TEF protein (TEF24) is aligned with that of CAR1. Identical amino acids are marked with a dash in the CAR1 sequence, and gaps in either sequence are indicated by spaces. The CRDs, which are discussed in the text, are marked with hatched bars. The putative death domains of both proteins are marked by a shaded bar.
In the putative extracellular domain, which is 82% identical to CAR1, there are 24 amino acid differences between the TEF protein and chicken CAR1. Ten of the amino acid differences lie within the membrane-distal region located before the first CRD, and 5 differences are within the membrane-proximal region positioned between the second CRD and the transmembrane domain. Within the two CRDs, there are 9 amino acid differences, including an additional cysteine (Cys-62) and an additional putative N-linked glycosylation site (Asn-Ser-Thr, residues 94–96) in the TEF protein. Significantly, the seven amino acid residues that are predicted to be important for the folding of the TNFR-like CRDs of CAR1 (5, 16) are absolutely conserved in the TEF protein (Tyr-67, Thr-82, Asn-98, Thr-99, Phe-108, Thr-123, and Asp-140).
While the cytoplasmic domain appears to be less well conserved between the TEF protein and CAR1, the putative death domains of these proteins are 75% identical. These identical residues include six (Phe-292, Arg-294, Leu-298, Glu-315, Trp-324, and Ile-353) of the TEF protein previously identified at corresponding positions in TNFR-I to be critical for cell killing (17).
The TEF Protein Is Expressed on the Cell Surface and Binds Specifically to an ALV-E SU Immunoadhesin.
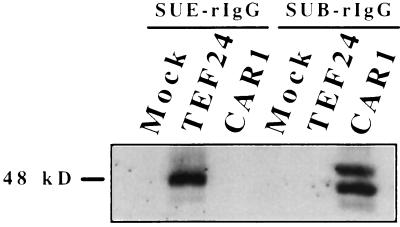
To determine whether the TEF protein is a subgroup E-specific viral receptor, we tested the ability of this protein to bind to ALV-E SU. To test this hypothesis, we constructed an immunoadhesin (SUE-rIgG) composed of a subgroup E-specific SU protein fused to the Fc region of a rabbit immunoglobulin, and this reagent was used like an antibody for immunoprecipitation and flow cytometry experiments. These experiments are similar to those used previously to study binding between ALV-B SU and CAR1 and between ALV-A SU and Tva, the receptor for subgroup A ALV (5, 11). SUE-rIgG specifically immunoprecipitated a protein from lysates of pBKTEF24-transfected 293 cells (Fig. 4, lane 2) but not from those of mock-transfected or pBK7.6–2-transfected CAR1-expressing cells (Fig. 4, lanes 1 and 3). This protein is similar in size to the two predominant CAR1 species precipitated by SUB-rIgG from lysates of pBK7.6–2-transfected 293 cells (Fig. 4, lane 6), which represent single- and double-glycosylated forms of CAR1 (H.B.A. and J.A.T.Y., unpublished data). SUB-rIgG did not precipitate the TEF protein (Fig. 4, lane 5) demonstrating that the turkey protein binds specifically to ALV-E SU.
Figure 4.
The TEF protein bound specifically to a subgroup E SU-immunoadhesin. Protein A-Sepharose beads bound to either SUB or SUE immunoadhesin were incubated with protein lysates from 293 cells transfected with either plasmid pBKTEF24 or plasmid pBK7.6–2 expressing CAR1 or with no DNA (mock). The immunoprecipitated protein was subjected to SDS/polyacrylamide gel electrophoresis and immunoblotted with the IQS579 CAR1 peptide antibody.
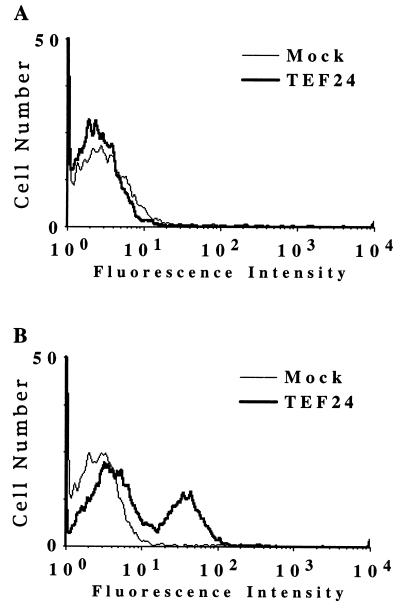
To test whether the TEF protein is expressed on the cell surface and whether ALV-E SU is capable of binding to the TEF protein when expressed in this location, flow cytometric analysis was performed on 293 cells transfected with pBKTEF24 (Fig. 5). Transfected 293 cells expressing the TEF protein and mock-transfected 293 cells were incubated with either SUE-rIgG or SUB-rIgG and then with fluorescein isothiocyanate (FITC)-conjugated antibodies specific for rabbit immunoglobulins. SUE-rIgG bound specifically to cells expressing the TEF protein (Fig. 5B), whereas SUB-rIgG did not (Fig. 5A). In addition, CAR1-expressing 293 cells did not bind to SUE-rIgG, although as expected they did bind to SUB-rIgG (data not shown). Therefore, these results demonstrated that the protein encoded by the pBKTEF24 cDNA clone is a cell surface receptor that binds specifically to ALV-E SU.
Figure 5.
The TEF protein is expressed on the cell surface and binds specifically to an ALV-E SU immunoadhesin. Human 293 cells transfected with plasmid pBKTEF24 or with no DNA (Mock) were bound to either SUB-rIgG (A) or SUE-rIgG (B), and flow cytometric analysis was performed as described previously (10). The pBKTEF24-expressing cells were specifically bound by SUE-rIgG but not by SUB-rIgG.
DISCUSSION
We have identified a turkey CAR1-related protein that confers susceptibility to infection by ALV-E, but not ALV-B, when expressed in human 293 cells. In addition, the turkey protein bound to a subgroup E-specific SU immunoadhesin. These two properties have demonstrated that this TEF protein is a subgroup E-specific viral receptor, and thus we have designated the protein as SEAR, for subgroup E ALV receptor. Given that Southern blot analysis indicate that there is a single gene highly related to CAR1 in turkey (J.B. and J.A.T.Y., unpublished data), it seems likely that SEAR is the turkey homologue of CAR1. These data provide direct evidence (previous evidence was indirect) that a subgroup E ALV receptor is structurally related to the receptor for viral subgroups B and D.
SEAR and CAR1 share significant amino acid similarity, being 89% identical in the two TNFR-like CRDs and 75% identical in the putative cytoplasmic death domain. One of the amino acid differences is an additional cysteine (Cys-62), in place of serine, in the first CRD of SEAR. The only other known example of a TNFR family member with two CRDs and a cytoplasmic death domain is the recently cloned human DR4 (18). DR4 is similar in amino acid composition to both CAR1 and SEAR, and it also has a cysteine residue located at a position corresponding to Cys-62 in SEAR. The natural ligand for DR4 was described previously as the orphan ligand TRAIL (18, 19), and it will be interesting to now determine if the avian ligands for SEAR and for CAR1 are related to TRAIL. Once the ligands for SEAR and CAR1 have been identified, we can compare how the molecular details of virus Env–receptor interactions relate to those of ligand–receptor interactions. An important outcome of this study will be to determine whether receptor interference following viral infection abrogates normal receptor function.
Mutational analysis of the extracellular regions of several retroviral receptors have identified important charged and aromatic amino acid residues essential for viral binding and entry (11, 20–22). For example, the extracellular domain of Tva, the receptor for ALV-A, contains two charged (Asp-46, Glu-47) and one aromatic (Trp-48) residue, in addition to a putative disulfide bond, which are important for entry of ALV-A (11, 20, 21). It will be of interest to determine whether similar types of residues are important for the function of CAR1 and SEAR. Because, the determinants of subgroup B viral entry lie exclusively within the extracellular domain of CAR1 (J.B., J.N., and J.A.T.Y., unpublished data), analysis of the 24 amino acid differences that distinguish this domain of CAR1 from SEAR should reveal binding and entry determinants for subgroup B, D, and E viruses.
We showed previously that CAR1 mediates apoptosis induced by binding the subgroup B SU immunoadhesin, a result which is consistent with the idea that Env–receptor interactions contribute to virus-induced cell death (5). This model for cell killing might explain why the determinants of Env that are required for subgroup B receptor usage correlate with those for cytopathicity (8). Therefore, the presence of a putative death domain in SEAR was unexpected, given that subgroup E viral infections are noncytopathic (6–8). Studies are now underway to determine whether SEAR is capable of signaling cell death, and if so, why ALV-E infection does not elicit that response.
Acknowledgments
We thank Connie Cepko and Mark Federspiel for providing proviruses and cells, and we thank John Daley at the Dana–Farber Cancer Institute for excellent assistance with the flow cytometric analysis. We also thank Derek Lindstrom and members of the Young laboratory for many stimulating discussions. This work was supported by National Institutes of Health Grant CA70810. M.M.R. is a predoctoral fellow of the Howard Hughes Medical Institute.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: Env, envelope protein; SU, viral surface; ALV, avian leukosis virus; CEFs, chicken embryo fibroblasts; TEFs, turkey embryo fibroblasts; CRDs, cysteine-rich domains; TNFR, tumor necrosis factor receptor; AP, alkaline phosphatase; SEAR, subgroup E ALV receptor.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF006002).
References
- 1.Weiss R A. In: The Retroviridae. Levy J A, editor; Levy J A, editor. New York: Plenum; 1993. pp. 1–107. [Google Scholar]
- 2.Hernandez L D, Hoffman L R, Wolfsberg T G, White J M. Annu Rev Cell Dev Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 3.Crittenden L B, Wendel E J, Motta J V. Virology. 1973;52:373–384. doi: 10.1016/0042-6822(73)90332-2. [DOI] [PubMed] [Google Scholar]
- 4.Crittenden L B, Motta J V. Virology. 1975;67:327–334. doi: 10.1016/0042-6822(75)90434-1. [DOI] [PubMed] [Google Scholar]
- 5.Brojatsch J, Naughton J, Rolls M M, Zingler Z, Young J A T. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 6.Weller S K, Joy A E, Temin H M. J Virol. 1980;33:494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weller S K, Temin H M. J Virol. 1981;39:713–721. doi: 10.1128/jvi.39.3.713-721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorner A J, Coffin J M. Cell. 1986;45:365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- 9.Young J A T, Bates P, Varmus H E. J Virol. 1993;67:1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates P, Young J A T, Varmus H E. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 11.Zingler K, Young J A T. J Virol. 1996;70:7510–7516. doi: 10.1128/jvi.70.11.7510-7516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields-Berry S C, Halliday A L, Cepko C L. Proc Natl Acad Sci USA. 1992;89:693–697. doi: 10.1073/pnas.89.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fekete D, Cepko C L. Mol Cell Biol. 1993;13:2604–2613. doi: 10.1128/mcb.13.4.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Heijne G. Nucl Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banner D W, D’Arcy A, Janes W, Gentz R, Schoenfeld H J, Broger C, Loetscher H, Lesslauer W. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- 17.Tartaglia L A, Ayres T M, Wong G H, Goeddel D V. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 18.Pan G, O’Rourke K, Chinnaiyan A M, Gentz R, Ebner R, Ni J, Dixit V M. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 19.Wiley S R, Schooley K, Smolak P J, Din W S, Huang C P, Nicholl J K, Sutherland G R, Smith T D, Rauch C, Smith C A, Goodwin R G. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 20.Bélanger C K, Zingler K, Young J A T. J Virol. 1993;69:1019–1024. doi: 10.1128/jvi.69.2.1019-1024.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zingler K, Bélanger C K, Peters D, Agard D, Young J A T. J Virol. 1995;69:4261–4266. doi: 10.1128/jvi.69.7.4261-4266.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhotra S, Scott A G, Zavorotinskaya T, Albritton L M. J Virol. 1996;70:321–326. doi: 10.1128/jvi.70.1.321-326.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]