Abstract
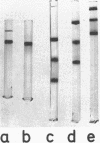
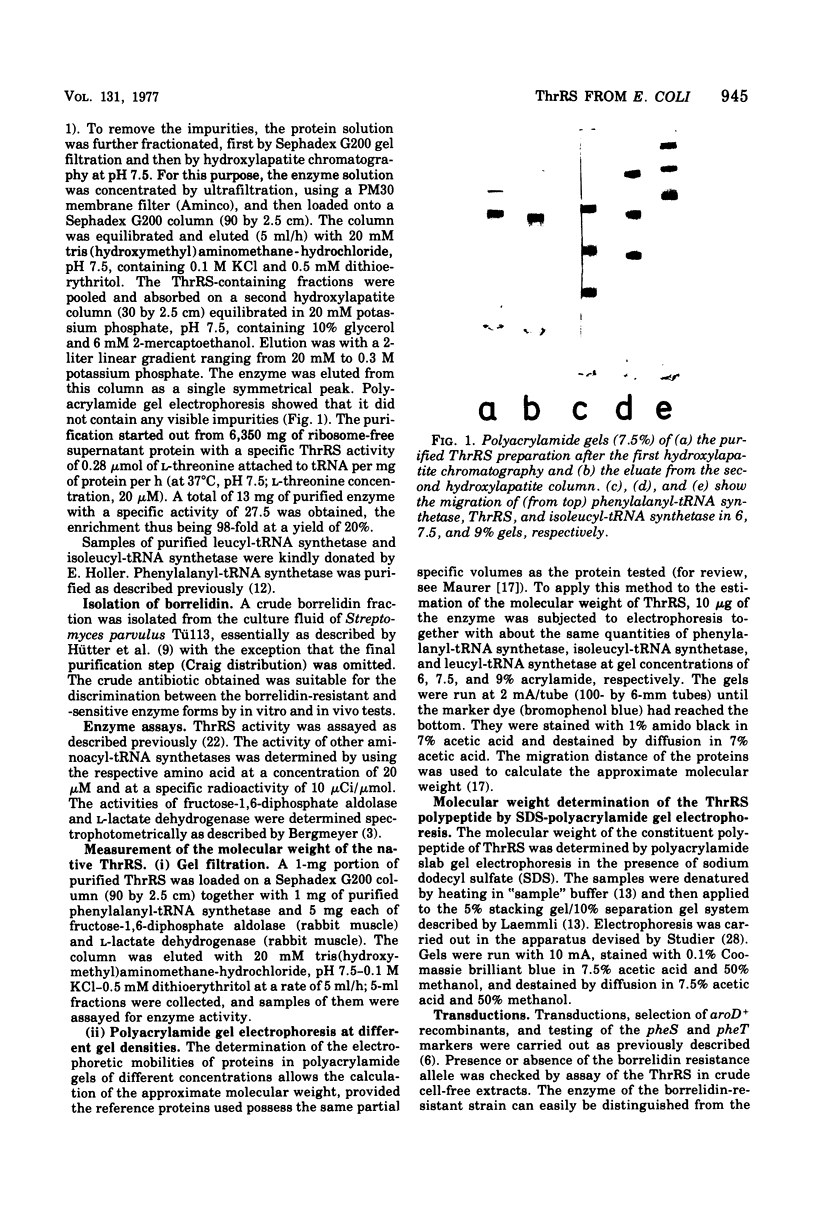
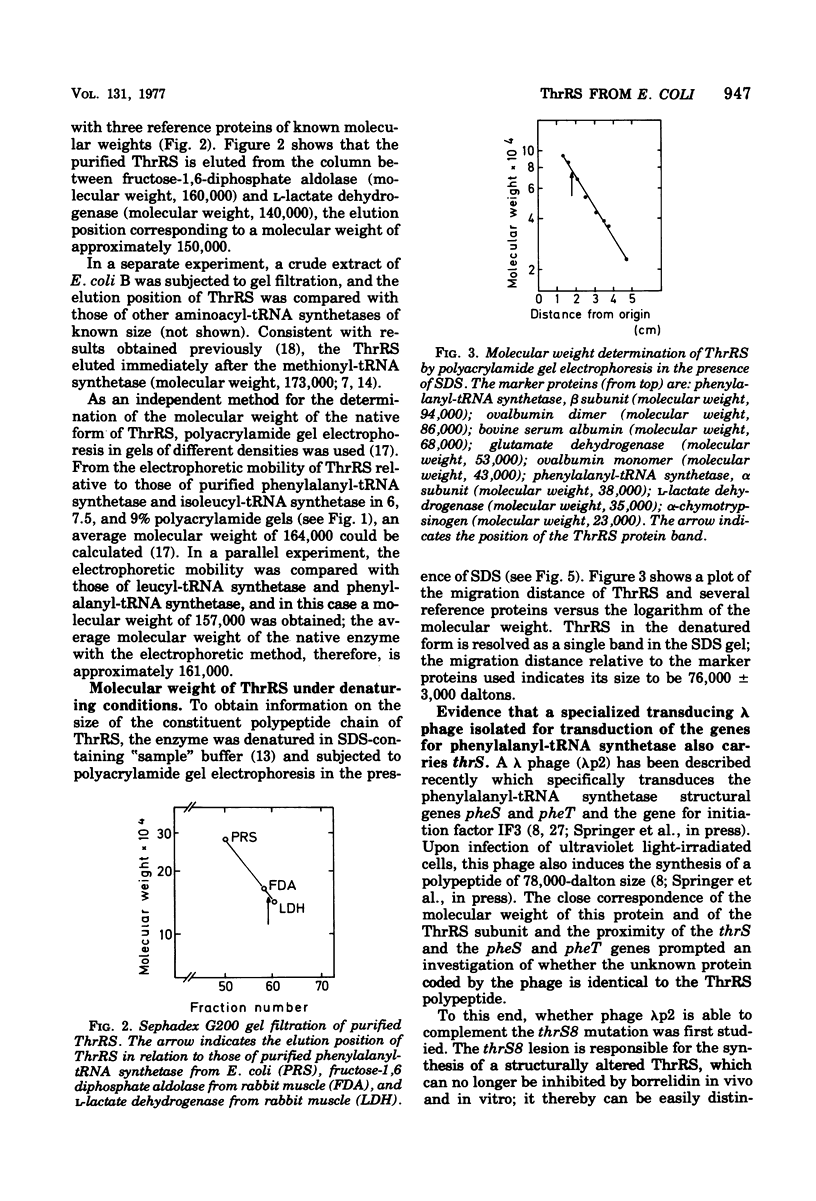
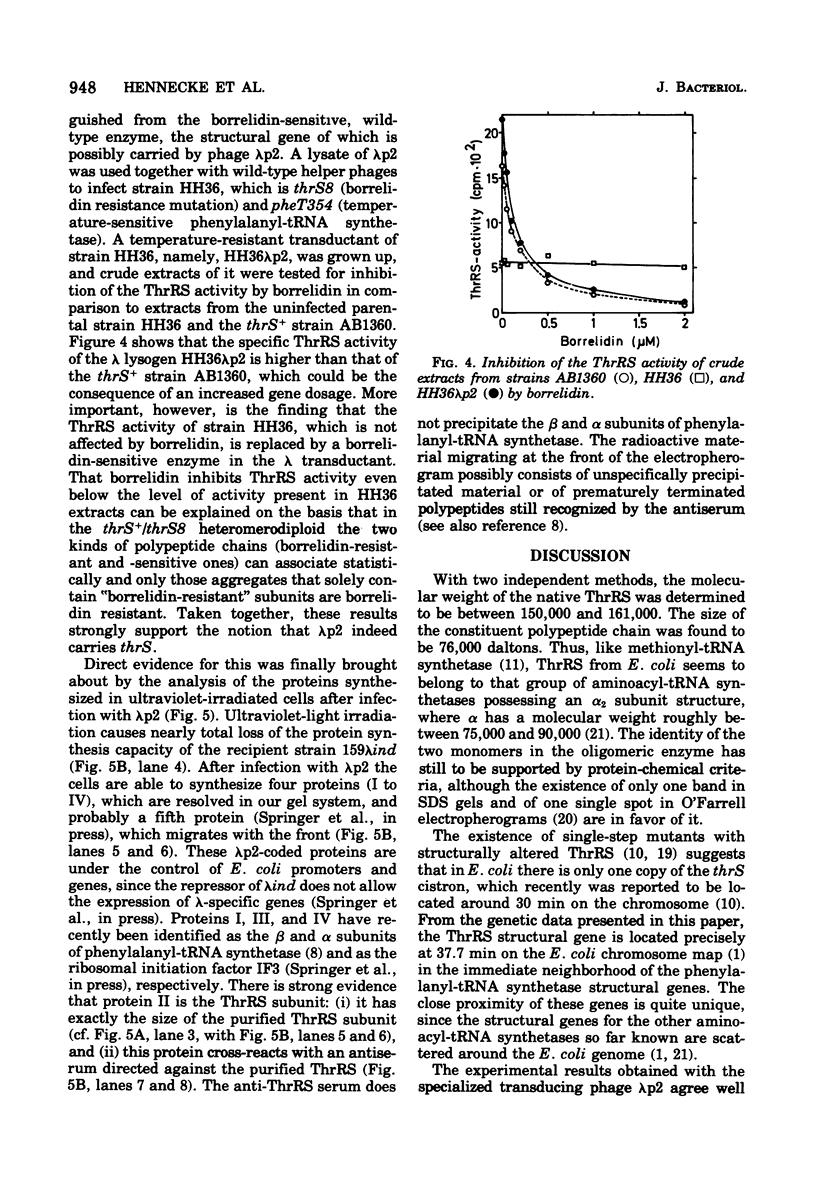
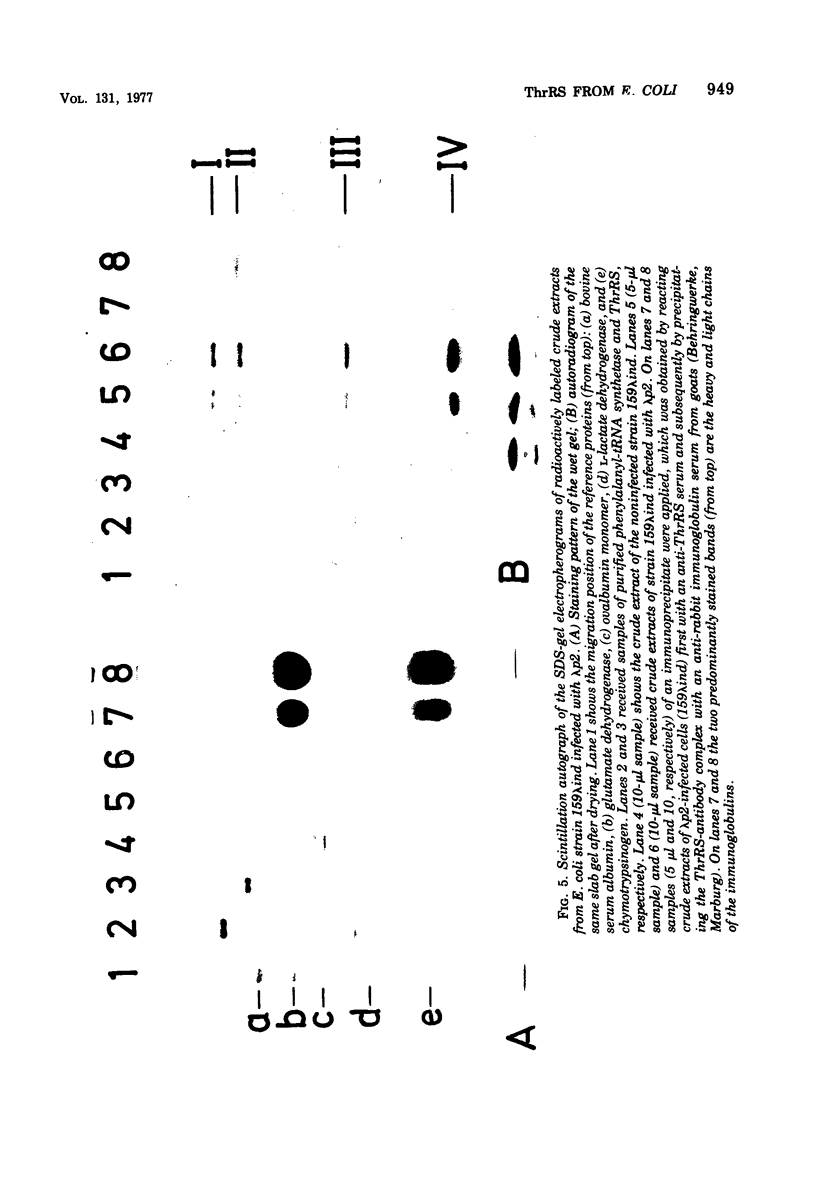
Threonyl-transfer ribonucleic acid synthetase (ThrRS) has been purified from a strain of Escherichia coli that shows a ninefold overproduction of this enzyme. Determination of the molecular weight of the purified, native enzyme by gel chromatography and by polyacrylamide gel electrophoresis at different gel concentrations yielded apparent molecular weight values of 150,000 and 161,000, respectively. Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate yields a single protein band of 76,000-dalton size. From these results an α2 subunit structure can be inferred. A mutant with a structurally altered ThrRS, which had been obtained by selection for resistance against the antibiotic borrelidin, was used to map the position of the ThrRS structural gene (thrS) by P1 transductions. It was found that thrS is located in the immediate neighborhood of pheS and pheT, which are the structural genes for the α and β subunits of phenylalanyl-transfer ribonucleic acid (tRNA) synthetase, the gene order being aroD-pheT-pheS-thrS. A λ phage that was previously shown to specifically transduce pheS, pheT, and also the structural gene for the translation initiation factor IF3 can complement the defect of the altered ThrRS of the borrelidin-resistant strain. This phage also stimulates the synthesis of the 76,000, molecular-weight polypeptide of ThrRS in ultraviolet light-irradiated. E. coli cells. These results indicate that the genes for ThrRS, α and β subunits of phenylalanyl-tRNA synthetase, and initiation factor IF3 are immediately adjacent on the E. coli chromosome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. FINE STRUCTURE OF A GENETIC REGION IN BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckel P. Identity of a gene responsible for suppression of aminoacyl-tRNA synthetase mutations with rpsT, the structural gene for ribosomal protein S20. Mol Gen Genet. 1976 Dec 8;149(2):225–228. doi: 10.1007/BF00332893. [DOI] [PubMed] [Google Scholar]
- Cassio D., Mathien Y., Waller J. P. Enhanced level and metabolic regulation of methionyl-transfer ribonucleic acid synthetase in different strains of Escherichia coli K-12. J Bacteriol. 1975 Aug;123(2):580–588. doi: 10.1128/jb.123.2.580-588.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer M. M., Böck A. Genes for the alpha and beta subunits of the phenylalanyl-transfer ribonucleic acid synthetase of Escherichia coli. J Bacteriol. 1976 Aug;127(2):923–933. doi: 10.1128/jb.127.2.923-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrikson R. L., Hartley B. S. Purification and properties of methionyl-transfer-ribonucleic acid synthetase from Escherichia coli. Biochem J. 1967 Oct;105(1):17–24. doi: 10.1042/bj1050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennecke H., Springer M., Böck A. A specialized transducing lambda phage carrying the Escherichia coli genes for phenylalanyl-tRNA synthetase. Mol Gen Genet. 1977 Apr 29;152(3):205–210. doi: 10.1007/BF00268819. [DOI] [PubMed] [Google Scholar]
- Hütter R., Poralla K., Zachau H. G., Zähner H. Stoffwechselprodukte von Mikroorganismen. 51. Uber die Wirkungsweise von Borrelidin-Hemmung des Threonineinbaus in sRNA. Biochem Z. 1966 Mar 28;344(2):190–196. [PubMed] [Google Scholar]
- Johnson E. J., Cohen G. N., Saint-Girons I. Threonyl-transfer ribonucleic acid synthetase and the regulation of the threonine operon in Escherichia coli. J Bacteriol. 1977 Jan;129(1):66–70. doi: 10.1128/jb.129.1.66-70.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G. L., Bruton C. J. The subunit structure of methionyl-tRNA synthetase from Escherichia coli. FEBS Lett. 1974 Mar 15;40(1):180–182. doi: 10.1016/0014-5793(74)80922-1. [DOI] [PubMed] [Google Scholar]
- Kosakowski M. H., Böck A. The subunit structure of phenylalanyl-tRNA synethetase of Escherichia coli. Eur J Biochem. 1970 Jan;12(1):67–73. doi: 10.1111/j.1432-1033.1970.tb00821.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemoine F., Waller J. P., van Rapenbusch R. Studies on methionyl transfer RNA synthetase. 1. Purification and some properties of methionyl transfer RNA synthetase from Escherichia coli K-12. Eur J Biochem. 1968 Apr 3;4(2):213–221. doi: 10.1111/j.1432-1033.1968.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass G., Thomale J. Alteration of structure of level of threonyl-tRNA-synthetase in Borrelidin resistant mutants of E. coli. FEBS Lett. 1974 Feb 15;39(2):182–186. doi: 10.1016/0014-5793(74)80046-3. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neihardt F. C., Parker J., McKeever W. G. Function and regulation of aminoacyl-tRNA synthetases in prokaryotic and eukaryotic cells. Annu Rev Microbiol. 1975;29:215–250. doi: 10.1146/annurev.mi.29.100175.001243. [DOI] [PubMed] [Google Scholar]
- Paetz W., Nass G. Biochemical and immunological characterization of threonyl-tRNA synthetase of two borrelidin-resistant mutants of Escherichia coli K12. Eur J Biochem. 1973 Jun;35(2):331–337. doi: 10.1111/j.1432-1033.1973.tb02843.x. [DOI] [PubMed] [Google Scholar]
- Piepersberg A., Hennecke H., Engelhard M., Nass G., Böck A. Cross-reactivity of phenylalanyl-transfer ribonucleic acid ligases from different microorganisms. J Bacteriol. 1975 Dec;124(3):1482–1488. doi: 10.1128/jb.124.3.1482-1488.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M., Graffe M., Grunberg-Manago M. Characterization of an E. coli mutant with a thermolabile initiation factor IF3 activity. Mol Gen Genet. 1977 Feb 28;151(1):17–26. doi: 10.1007/BF00446908. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]