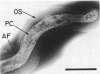
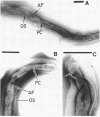
Abstract
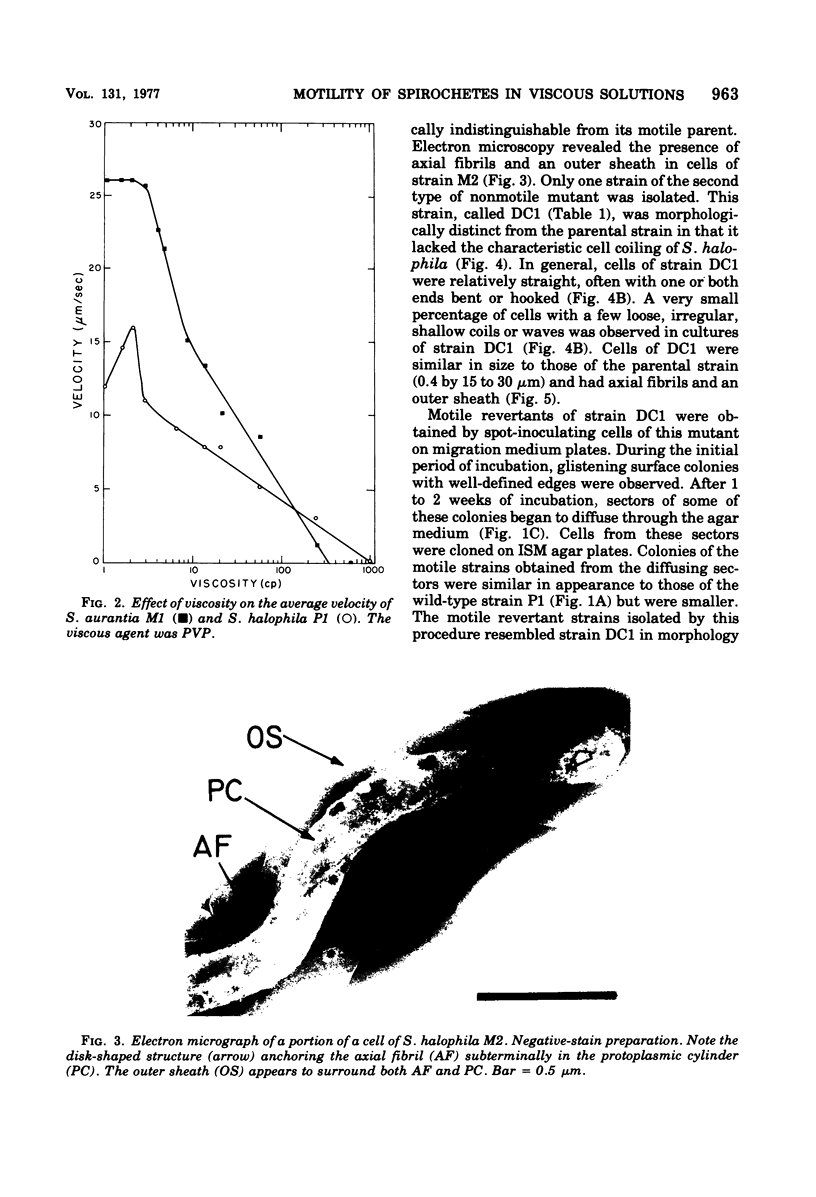
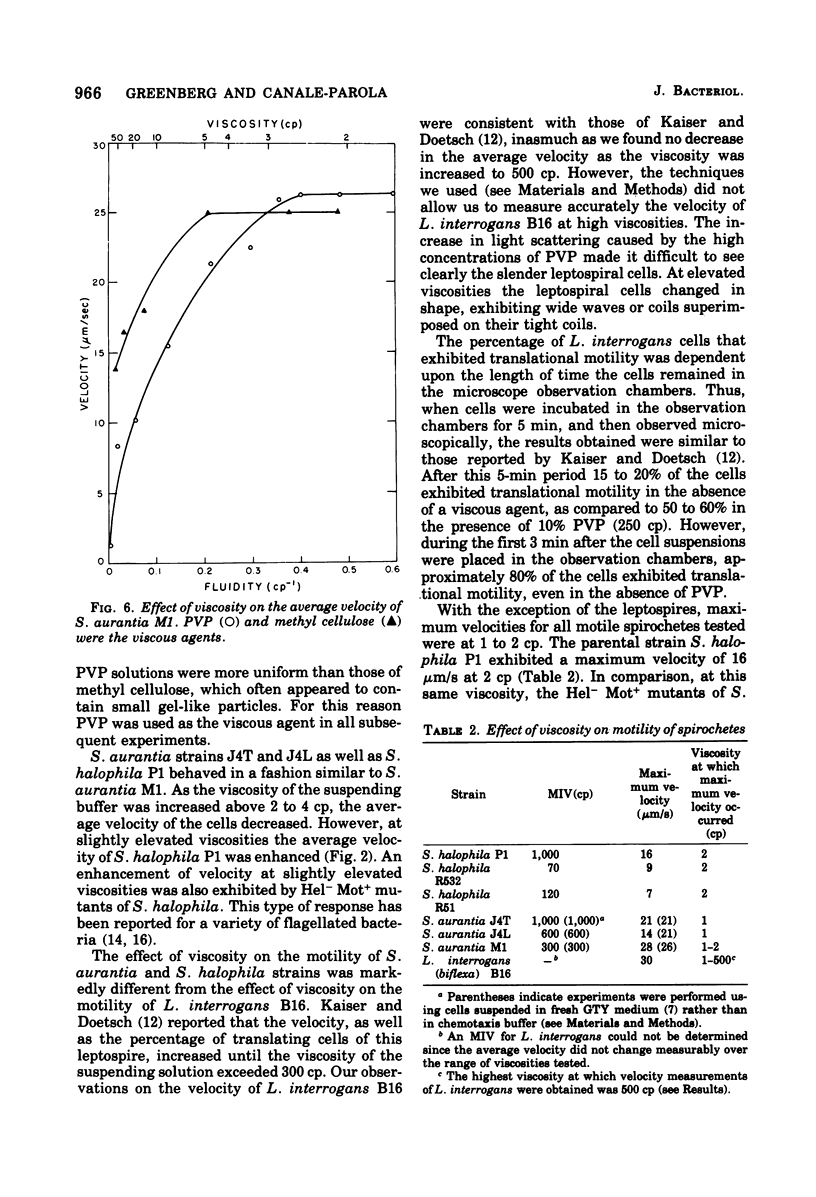
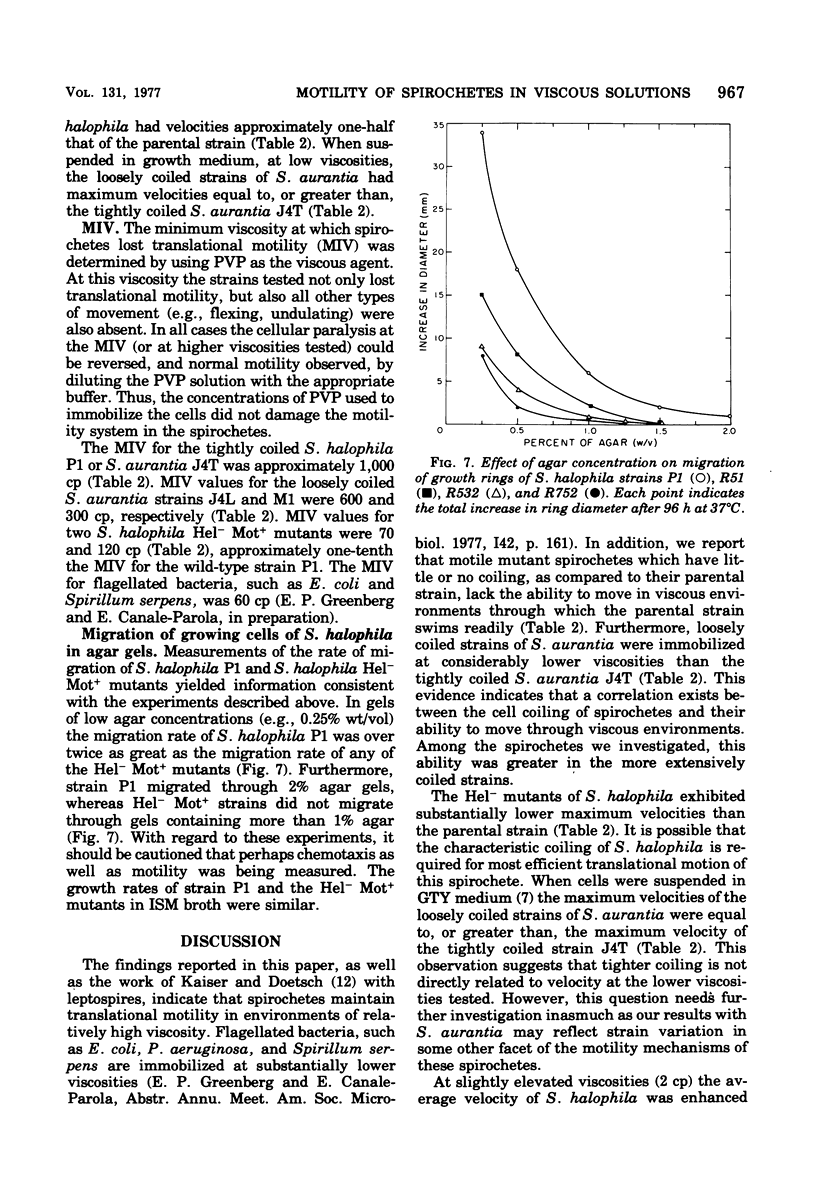
The lowest viscosity that stops translational motility of cells (minimum immobilizing viscosity [MIV] was determined for various spirochetes. The viscous agent used was polyvinylpyrrolidone, The MIV for either Spirochaeta halophila P1 or Spirochaeta aurantia J4T was approximately 1,000 centipoise (cp), and for Leptospira interrogans (biflexa) B16 the MIV was greater than 500 cp. In comparison, the MIV for the flagellated bacteria Escherichia coli and Spirillum serpens was 60 cp. MIV values for two S. halophila mutant strains lacking the characteristic cell coiling (Hel-mutants) were 70 and 120 cp, approximately one-tenth the MIV for the wild-type strain. MIV values for cells of S. aurantia strains with fewer coils than comparably long cells of S. aurantia J4T were 300 to 600 cp. The average velocity of strains of S. aurantia and S. halophila decreased at viscosities higher than 2 to 3 cp. At 2 cp the average velocity of S. halophila P1 was 16 micron/s, whereas the average velocities of Hel-mutant strains were 7 to 9 micron/s. This study indicates that the coiling of spirochetes plays a role in their ability to move through environments of realtively high viscosity. Among the spirochetes we investigated, this ability is greater in the more extensively coiled strains.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg H. C. How spirochetes may swim. J Theor Biol. 1976 Feb;56(2):269–273. doi: 10.1016/s0022-5193(76)80074-4. [DOI] [PubMed] [Google Scholar]
- Blakemore R. P., Canale-Parola E. Morphological and ecological characteristics of Spirochaeta plicatilis. Arch Mikrobiol. 1973;89(4):273–289. doi: 10.1007/BF00408895. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Canale-Parola E. Morphology and physiology of Spirochaeta aurantia strains isolated from aquatic habitats. Arch Microbiol. 1975 Sep 30;105(1):1–12. doi: 10.1007/BF00447104. [DOI] [PubMed] [Google Scholar]
- Cox P. J., Twigg G. I. Leptospiral motility. Nature. 1974 Jul 19;250(463):260–261. doi: 10.1038/250260a0. [DOI] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Chemotaxis in Spirochaeta aurantia. J Bacteriol. 1977 Apr;130(1):485–494. doi: 10.1128/jb.130.1.485-494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Spirochaeta halophila sp. n., a facultative anaerobe from a high-salinity pond. Arch Microbiol. 1976 Nov 2;110(23):185–194. doi: 10.1007/BF00690227. [DOI] [PubMed] [Google Scholar]
- Henneberry R. C., Baseman J. B., Cox C. D. Growth of a water strain of Leptospira in synthetic media. Antonie Van Leeuwenhoek. 1970;36(4):489–501. doi: 10.1007/BF02069051. [DOI] [PubMed] [Google Scholar]
- JAHN T. L., LANDMAN M. D. LOCOMOTION OF SPIROCHETES. Trans Am Microsc Soc. 1965 Jul;84:395–406. [PubMed] [Google Scholar]
- Joseph R., Canale-Parola E. Axial fibrils of anaerobic spirochetes: ultrastructure and chemical characteristics. Arch Mikrobiol. 1972;81(2):146–168. doi: 10.1007/BF00412325. [DOI] [PubMed] [Google Scholar]
- Joseph R., Holt S. C., Canale-Parola E. Peptidoglycan of free-living anaerobic spirochetes. J Bacteriol. 1973 Jul;115(1):426–435. doi: 10.1128/jb.115.1.426-435.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser G. E., Doetsch R. N. Letter: Enhanced translational motion of Leptospira in viscous environments. Nature. 1975 Jun 19;255(5510):656–657. doi: 10.1038/255656a0. [DOI] [PubMed] [Google Scholar]
- Kehry M., Yguerabide J., Singer S. J. Fluidity in the membranes of adult and neonatal human erythrocytes. Science. 1977 Feb 4;195(4277):486–487. doi: 10.1126/science.835005. [DOI] [PubMed] [Google Scholar]
- Schneider W. R., Doetsch R. N. Effect of viscosity on bacterial motility. J Bacteriol. 1974 Feb;117(2):696–701. doi: 10.1128/jb.117.2.696-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S. J., Cooper R. A. Membrane microviscosity and human platelet function. Biochemistry. 1976 Nov 2;15(22):4832–4837. doi: 10.1021/bi00667a012. [DOI] [PubMed] [Google Scholar]
- Staneck J. L., Henneberry R. C., Cox C. D. Growth requirements of pathogenic Leptospira. Infect Immun. 1973 Jun;7(6):886–897. doi: 10.1128/iai.7.6.886-897.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. A., Kalan J. Intracellular Treponema pallidum in cells of a syphilitic lesion of the uterine cervix. Am J Obstet Gynecol. 1975 Jun 1;122(3):361–367. doi: 10.1016/0002-9378(75)90185-4. [DOI] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N., Kalan A. J. Treponema pallidum within cells of a primary chancre from a human female. Br J Vener Dis. 1974 Feb;50(1):40–44. doi: 10.1136/sti.50.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]