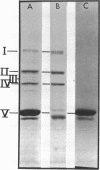
Abstract
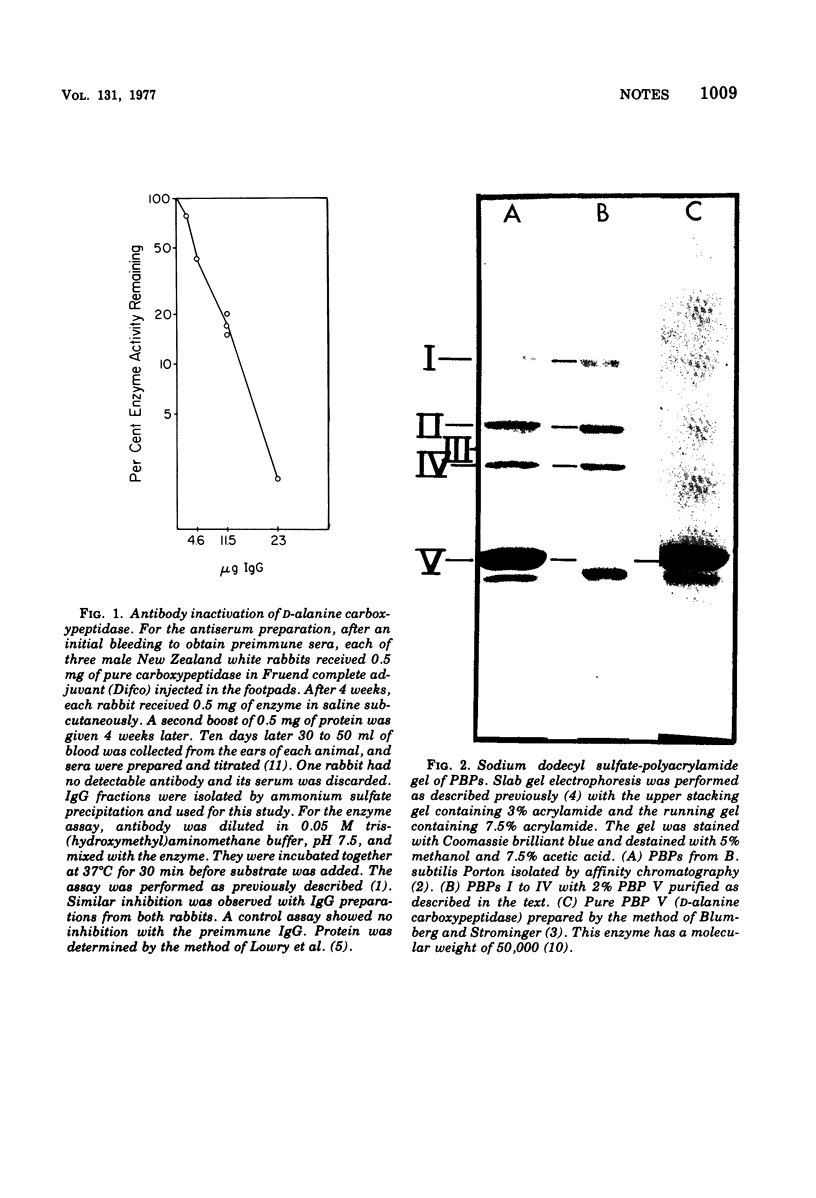
The fact that antibody to d-alanine carboxypeptidase of Bacillus subtilis does not cross-react with other penicillin-binding proteins suggests that these proteins are not precursors or multimers of the enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Covalent affinity chromatography of penicillin-binding components from bacterial membranes. Methods Enzymol. 1974;34:401–405. doi: 10.1016/s0076-6879(74)34046-3. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Inactivation of D-alanine carboxypeptidase by penicillins and cephalosporins is not lethal in Bacillus subtilis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2814–2817. doi: 10.1073/pnas.68.11.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Isolation by covalent affinity chromatography of the penicillin-binding components from membranes of Bacillus subtilis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3751–3755. doi: 10.1073/pnas.69.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Strominger J. L. Altered penicillin-binding components in penicillin-resistant mutants of Bacillus subtilis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1816–1820. doi: 10.1073/pnas.73.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suginaka H., Blumberg P. M., Strominger J. L. Multiple penicillin-binding components in Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5279–5288. [PubMed] [Google Scholar]
- Umbreit J. N., Strominger J. L. D-alanine carboxypeptidase from Bacillus subtilis membranes. I. Purification and characterization. J Biol Chem. 1973 Oct 10;248(19):6759–6766. [PubMed] [Google Scholar]
- WADSWORTH C. A slide microtechnique for the analysis of immune precipitates in gel. Int Arch Allergy Appl Immunol. 1957;10(6):355–360. doi: 10.1159/000228394. [DOI] [PubMed] [Google Scholar]