Abstract
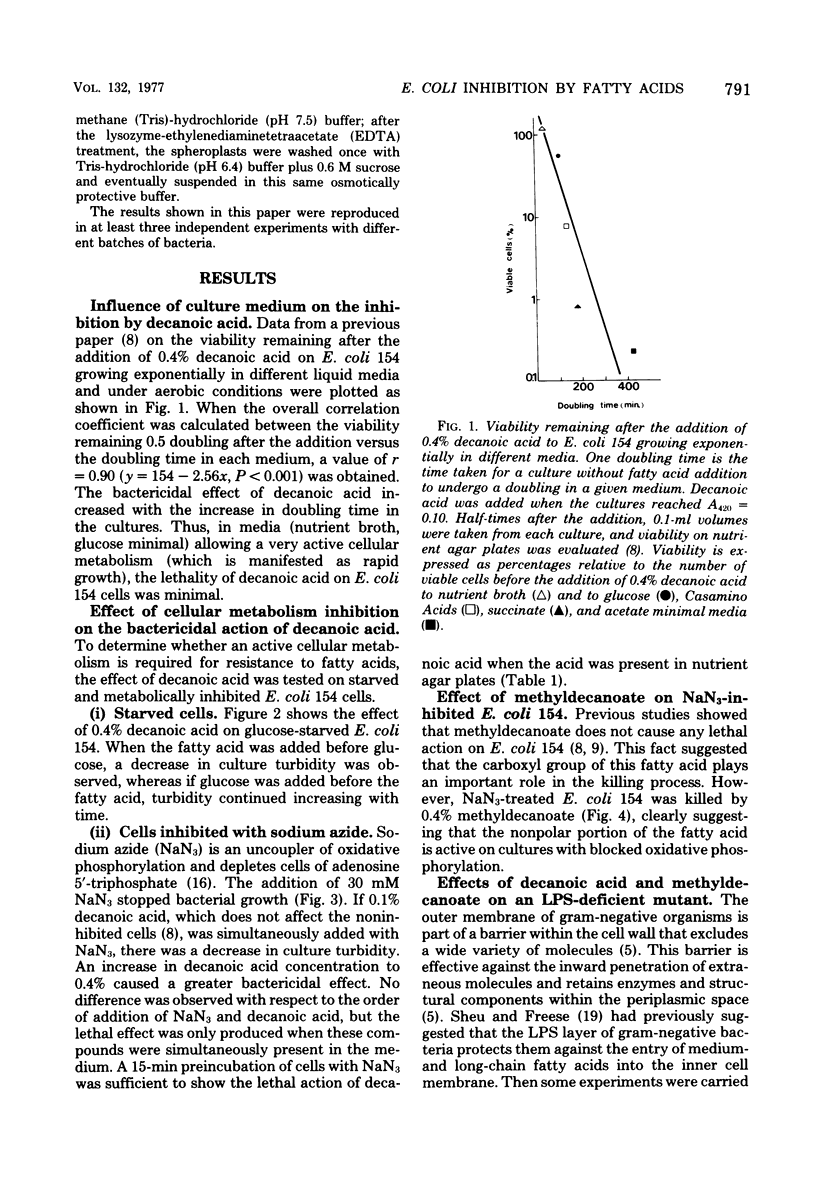
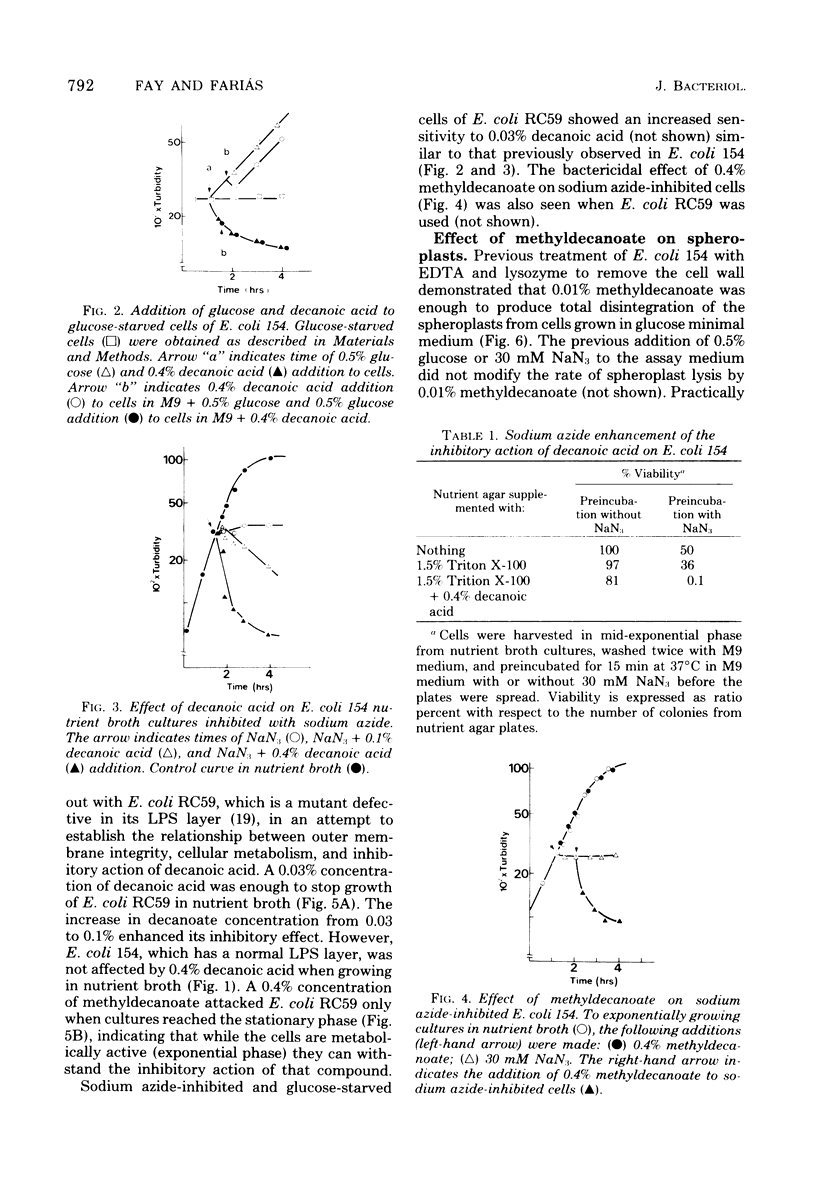
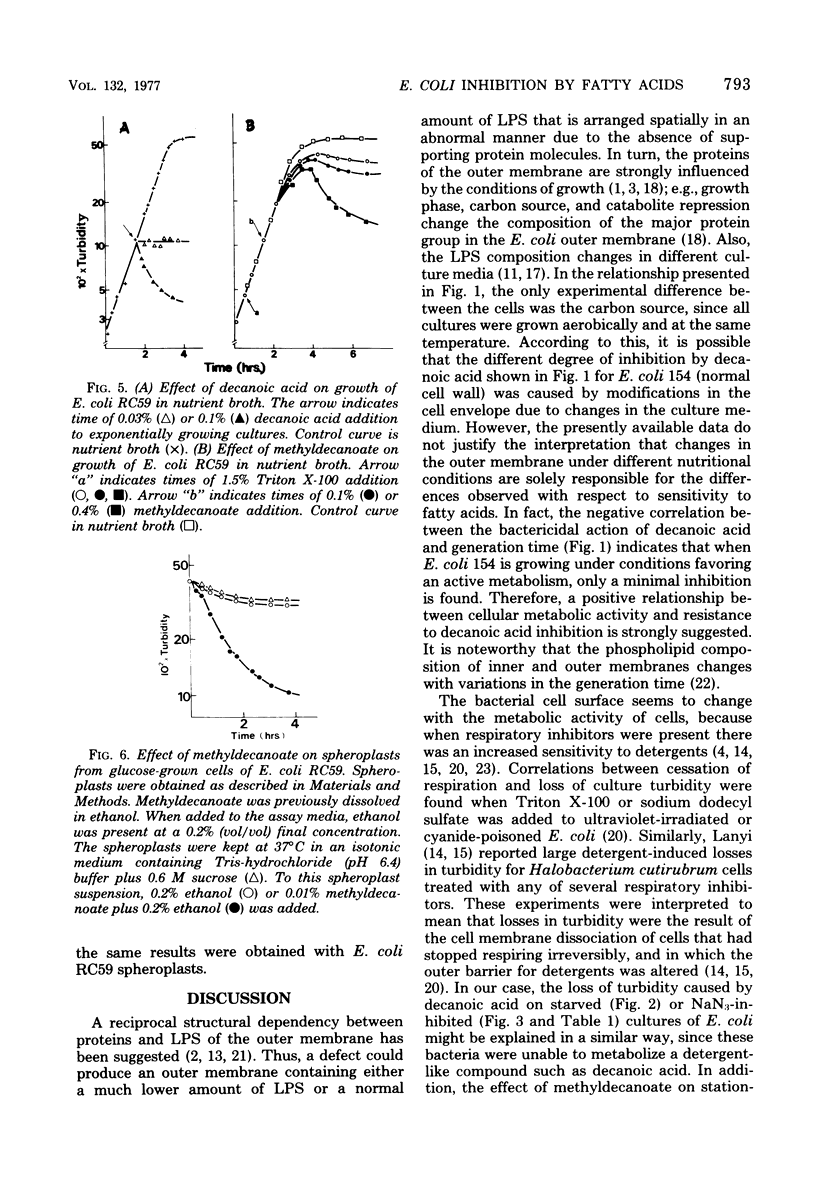
The inhibitory action of decanoic acid on both Escherichia coli K-12/154 (normal lipopolysaccharide) and E. coli RC59 (defective lipopolysaccharide) was studied. A correlation was found between the doubling time of E. coli 154 growing in different media and the lethal effect of 0.4% decanoic acid on this bacterium. Decanoic acid (0.4%) exerted a lytic action on glucose-starved and NaN3-inhibited cells of E. coli 154 and RC59. Exponentially growing cultures of both strains were not affected by the addition of 0.4% methyldecanoate, but cells of E. coli RC59 reaching the stationary phase were attacked by that compound. A bactericidal action of 0.4% methyldecanoate on exponential E. coli 154 and RC59 was observed when sodium azide was also present in the media. Concentrations lower than 0.01% methyldecanoate had a lytic effect on spheroplasts from E. coli 154 and RC59. These results indicate that the inhibitory action of a non-metabolizable fatty acid on E. coli depends on the cellular metabolic activity and the outer membrane integrity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLLE A., KELLENBERGER E. Etude de l'action du laurylsulfate de sodium sur E. coli. Schweiz Z Pathol Bakteriol. 1958;21(3):714–740. [PubMed] [Google Scholar]
- Bennett R. L., Rothfield L. I. Genetic and physiological regulation of intrinsic proteins of the outer membrane of Salmonella typhimurium. J Bacteriol. 1976 Jul;127(1):498–504. doi: 10.1128/jb.127.1.498-504.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Dissociation and reassembly of Escherichia coli outer membrane and of lipopolysaccharide, and their reassembly onto flagellar basal bodies. J Bacteriol. 1971 Mar;105(3):1184–1199. doi: 10.1128/jb.105.3.1184-1199.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr Membrane adenosine triphosphatase of Escherichia coli: activation by calcium ion and inhibition by monovalent cations. J Bacteriol. 1969 Nov;100(2):914–922. doi: 10.1128/jb.100.2.914-922.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay J. P., Farias R. N. The inhibitory action of fatty acids on the growth of Escherichia coli. J Gen Microbiol. 1975 Dec;91(2):233–240. doi: 10.1099/00221287-91-2-233. [DOI] [PubMed] [Google Scholar]
- Fay J. P., Farías R. N. Chilling cells enhances the bactericidal action of fatty acids on Escherichia coli. Appl Environ Microbiol. 1976 Feb;31(2):153–157. doi: 10.1128/aem.31.2.153-157.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith H., Miller T. B. Physicochemical effects of long chain fatty acids on bacterial cells and their protoplasts. J Appl Bacteriol. 1973 Dec;36(4):647–658. doi: 10.1111/j.1365-2672.1973.tb04150.x. [DOI] [PubMed] [Google Scholar]
- Hirt W. E., Vestal J. R. Physical and chemical studies of Thiobacillus ferroxidans lipopolysaccharides. J Bacteriol. 1975 Aug;123(2):642–650. doi: 10.1128/jb.123.2.642-650.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo E., Kanai K. Further studies on the lethal effect of long-chain fatty acids on mycobacteria. Jpn J Med Sci Biol. 1976 Feb;29(1):25–37. doi: 10.7883/yoken1952.29.25. [DOI] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Influence of electron transport on the interaction between membrane lipids and Triton X-100 in Halobacterium cutirubrum. Biochemistry. 1973 Mar 27;12(7):1433–1438. doi: 10.1021/bi00731a025. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. 8. Respiration-dependent detergent dissolution of cell envelopes. Biochim Biophys Acta. 1972 Sep 1;282(1):439–446. doi: 10.1016/0005-2736(72)90351-3. [DOI] [PubMed] [Google Scholar]
- Nazar R. N., Wong J. T. Inhibitor-induced shift-downs in Escherichia coli. J Bacteriol. 1969 Nov;100(2):956–961. doi: 10.1128/jb.100.2.956-961.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin A. M., Kalckar H. M., Alberico L. The metabolic basis for masking of receptor-sites on E. coli K-12 for C21, a lipopolysaccharide core-specific phage. Arch Biochem Biophys. 1968 Oct;128(1):95–105. doi: 10.1016/0003-9861(68)90011-8. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Lipopolysaccharide layer protection of gram-negative bacteria against inhibition by long-chain fatty acids. J Bacteriol. 1973 Sep;115(3):869–875. doi: 10.1128/jb.115.3.869-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L. Evidence relating cessation of respiration, cell envelope changes, and death in ultraviolet-irradiated Escherichia coli B-r cells. J Bacteriol. 1974 Feb;117(2):551–559. doi: 10.1128/jb.117.2.551-559.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., Glauert A. M. Synthesis and turnover of the regularly arranged surface protein of Acinetobacter sp. relative to the other components of the cell envelope. J Bacteriol. 1976 Jul;127(1):440–450. doi: 10.1128/jb.127.1.440-450.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwinkel E., De Vlieghere M., Fontaine M., Charles D., Denamur F., Vandevoorde D., De Kegel D. Septation deficiency and phosphilipid perturbation in Escherichia coli genetically constitutive for the beta oxidation pathway. J Bacteriol. 1976 Sep;127(3):1389–1399. doi: 10.1128/jb.127.3.1389-1399.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldringh C. L., van Iterson W. Effects of treatment with sodium dodecyl sulfate on the ultrastructure of Escherichia coli. J Bacteriol. 1972 Sep;111(3):801–813. doi: 10.1128/jb.111.3.801-813.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



