Abstract
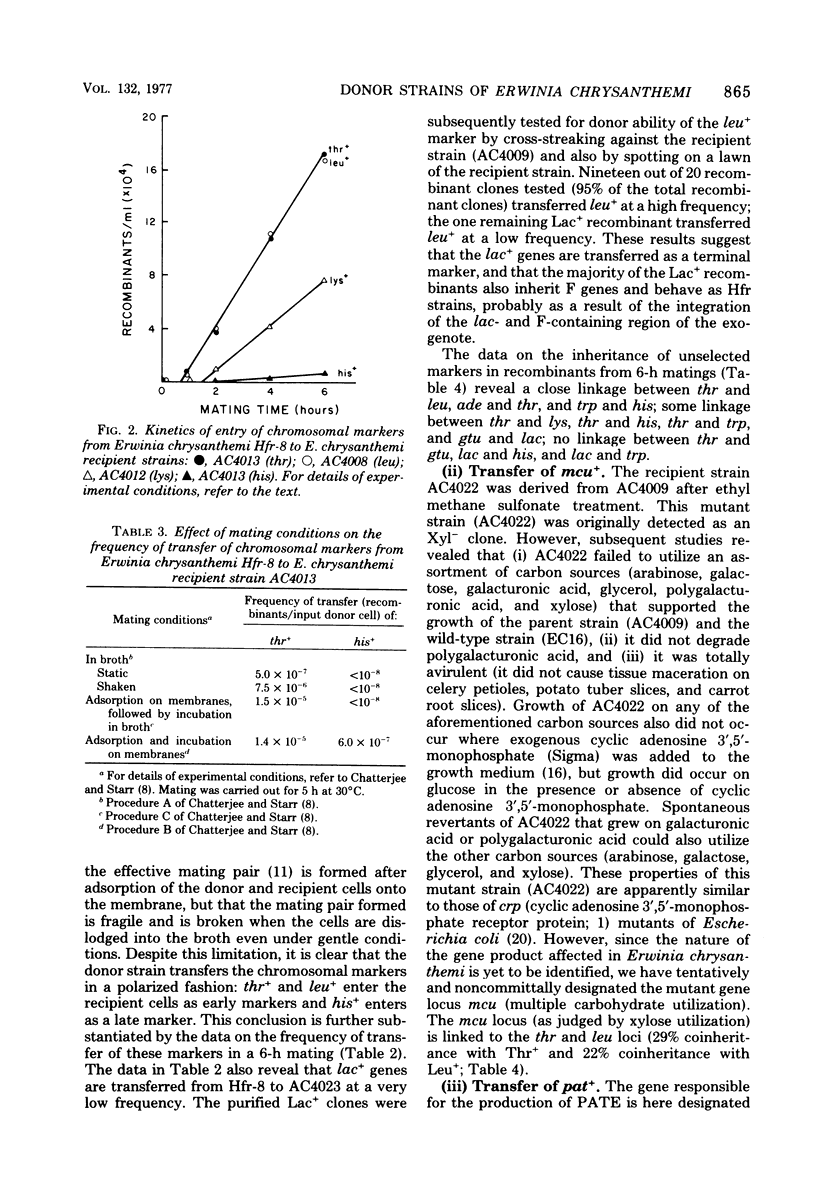
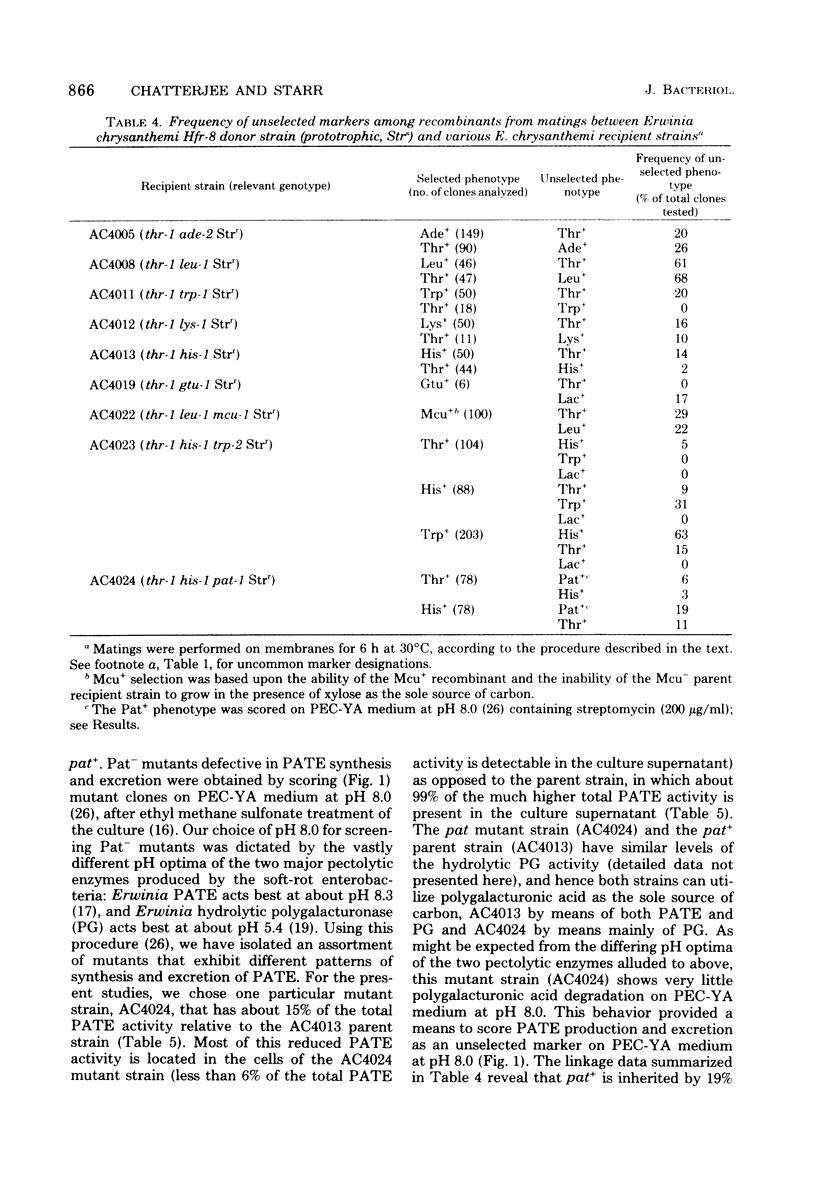
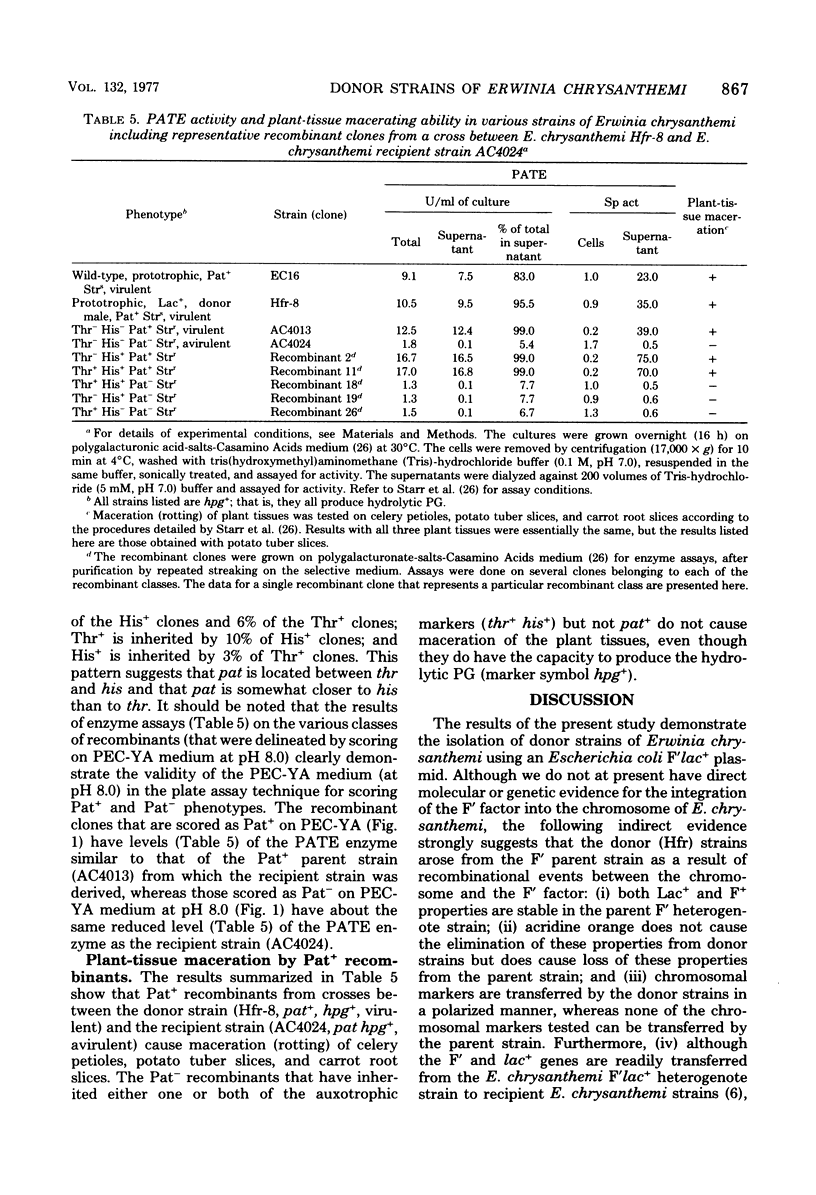
Donor strains of Erwinia chrysanthemi ICPB EC16, a member of the soft-rot (pectolytic) section of the enterobacterial genus Erwinia, were obtained by chromosomal integration of an F′lac+ plasmid originating from Escherichia coli. These stable donor strains, selected from an unstable F′lac+ heterogenote by repeated platings of single Lac+ colonies on lactose minimal agar, do not segregate (as does the parent F′lac+ heterogenote) into Lac− or F− clones, in either the presence or absence of acridine orange. One representative donor strain (from the 12 that have been selected) has been examined in more detail; it can transfer ade+, gal+, gtu+ (utilization of galacturonate), his+, lac+, leu+, lys+, mcu+ (multiple carbohydrate utilization), pat+ (production of polygalacturonic acid trans-eliminase), thr+, and trp+ in a polarized manner to appropriate recipient strains of E. chrysanthemi; the frequencies of ade+, leu+, and thr+ transfer were higher than those of the other markers tested to date. This donor strain transfers lac+ genes during a 6-h mating on membranes; most of the Lac+ recombinants are donors of chromosomal markers. The kinetics of entry as well as the frequencies of transfer of chromosomal markers indicate that thr+ and leu+ enter the recipient as proximal markers and that lac+ enters as a distal marker. Analysis of the recombinants demonstrates close linkage between thr and leu, ade and thr, his and pat, and his and trp loci. The results suggest that the integration of F′lac+ into the chromosome of E. chrysanthemi has occurred at a region adjacent to the leu-thr loci, and that the chromosome is transferred in the following sequence: origin----leu--thr--ade--lys--mcu--pat--his--trp--gal--gtu--lac--F. Plant-tissue maceration occurs in Pat+ recombinants and not in Pat− recombinants, even though both form another pectolytic enzyme, hydrolytic polygalacturonase. This genetic evidence supports the idea that the E. chrysanthemi polygalacturonic acid trans-eliminase plays an essential role in bringing about plant-tissue maceration.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Buss R. F., Starr M. P. Unusual susceptibility of Erwinia amylovora to antibacterial agents in relation to the barrier function of its cell envelope. Antimicrob Agents Chemother. 1977 May;11(5):897–905. doi: 10.1128/aac.11.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Gene transmission among strains of Erwinia amylovora. J Bacteriol. 1973 Dec;116(3):1100–1106. doi: 10.1128/jb.116.3.1100-1106.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Genetic transfer of episomic elements among Erwinia species and other enterobacteria: F'Lac+. J Bacteriol. 1972 Jul;111(1):169–176. doi: 10.1128/jb.111.1.169-176.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Transfer among Erwinia spp. and other enterobacteria of antibiotic resistance carried on R factors. J Bacteriol. 1972 Oct;112(1):576–584. doi: 10.1128/jb.112.1.576-584.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Transmission of lac by the sex factor E in Erwinia strains from human clinical sources. Infect Immun. 1973 Oct;8(4):563–572. doi: 10.1128/iai.8.4.563-572.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- Moran F., Nasuno S., Starr M. P. Extracellular and intracellular polygllacturonic acid trans-eliminases of Erwinia carotovora. Arch Biochem Biophys. 1968 Feb;123(2):298–306. doi: 10.1016/0003-9861(68)90138-0. [DOI] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonase of Erwinia carotovora. J Biol Chem. 1966 Nov 25;241(22):5298–5306. [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugashetti B. K., Starr M. P. Conjugational transfer of genes determining plant virulence in Erwinia amylovora. J Bacteriol. 1975 May;122(2):485–491. doi: 10.1128/jb.122.2.485-491.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Genetic relatedness in the family Enterobacteriaceae. Annu Rev Microbiol. 1976;30:327–349. doi: 10.1146/annurev.mi.30.100176.001551. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Ross H., Ziegler L., Mäkelä P. H. F + , Hfr, and F' strains of Salmonella typhimurium and Salmonella abony. Bacteriol Rev. 1972 Dec;36(4):608–637. doi: 10.1128/br.36.4.608-637.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
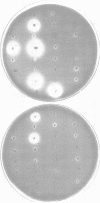
- Starr M. P., Chatterjee A. K., Starr P. B., Buchanan G. E. Enzymatic degradation of polygalacturonic acid by Yersinia and Klebsiella species in relation to clinical laboratory procedures. J Clin Microbiol. 1977 Oct;6(4):379–386. doi: 10.1128/jcm.6.4.379-386.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr M. P., Chatterjee A. K. The genus Erwinia: enterobacteria pathogenic to plants and animals. Annu Rev Microbiol. 1972;26:389–426. doi: 10.1146/annurev.mi.26.100172.002133. [DOI] [PubMed] [Google Scholar]
- de Graaff J., Kreuning C., Stouthamer A. H. Isolation and characterization of Hfr males in Citrobacter freundii. Antonie Van Leeuwenhoek. 1974;40(1):161–170. doi: 10.1007/BF00394563. [DOI] [PubMed] [Google Scholar]