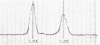Abstract
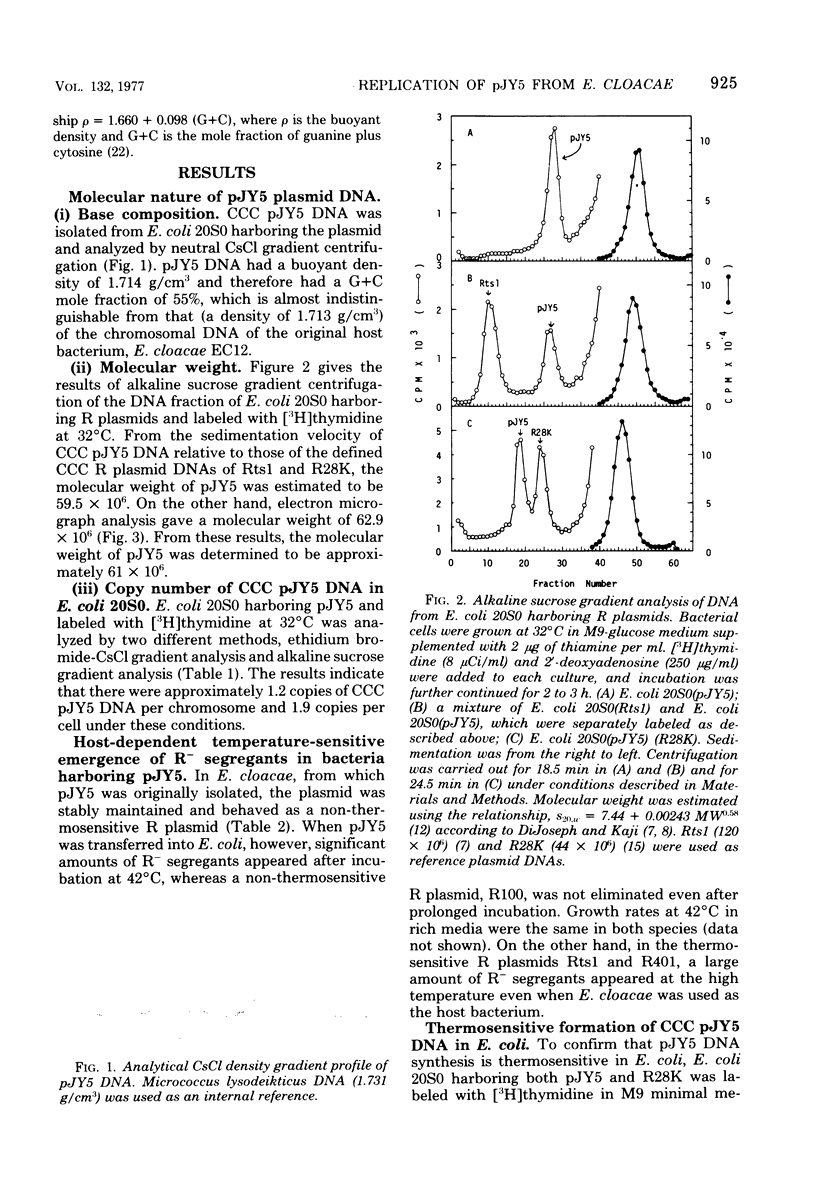
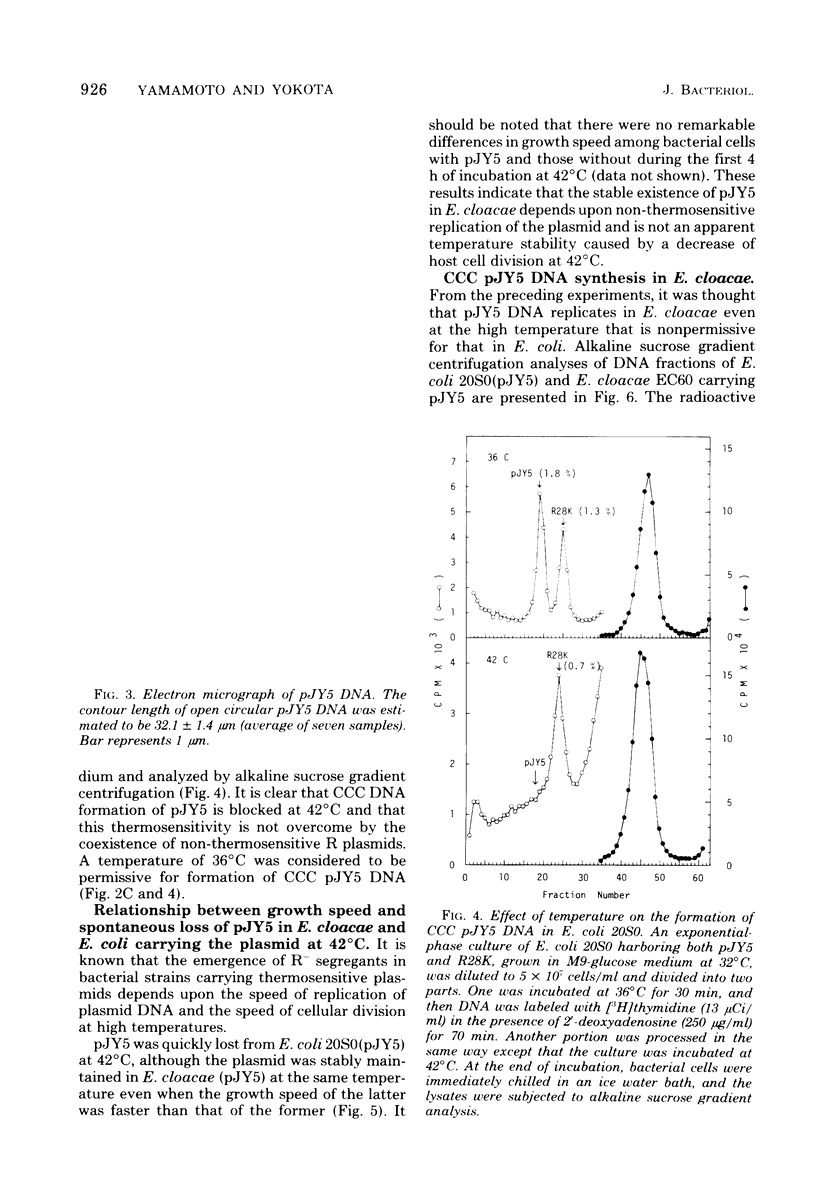
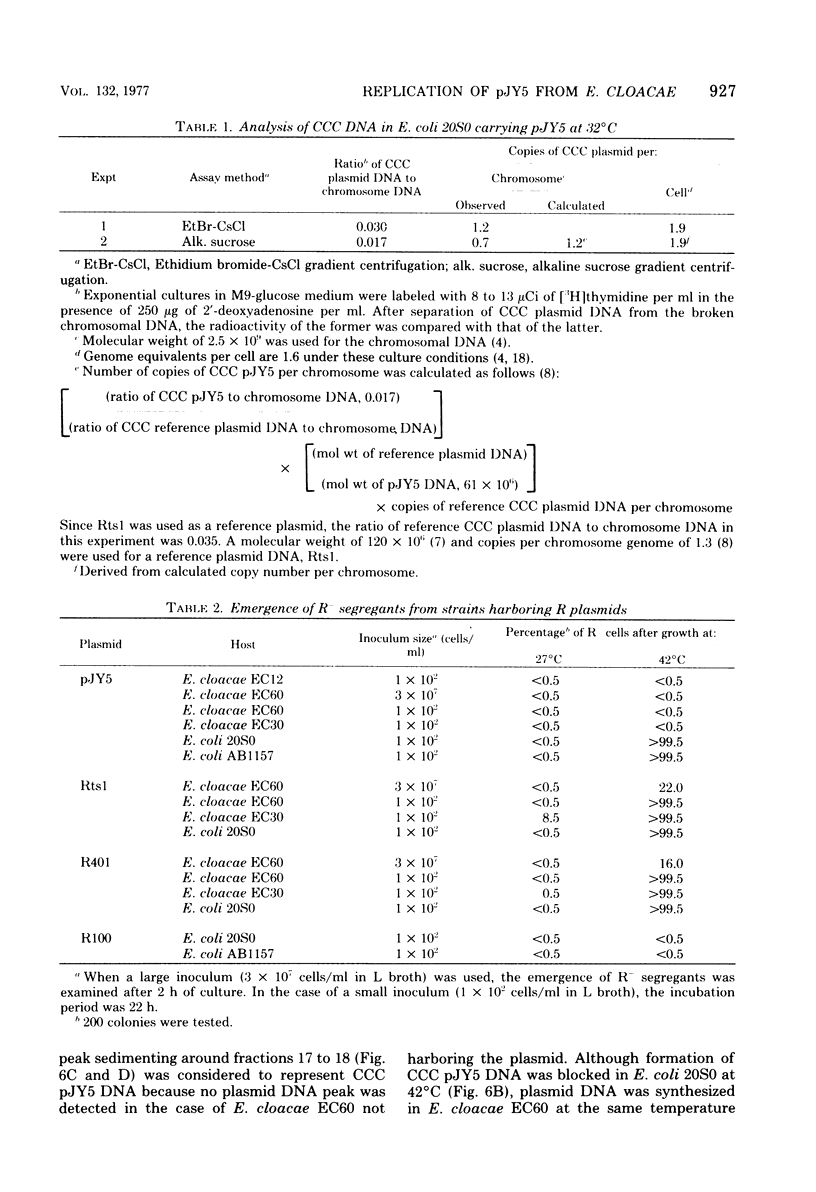
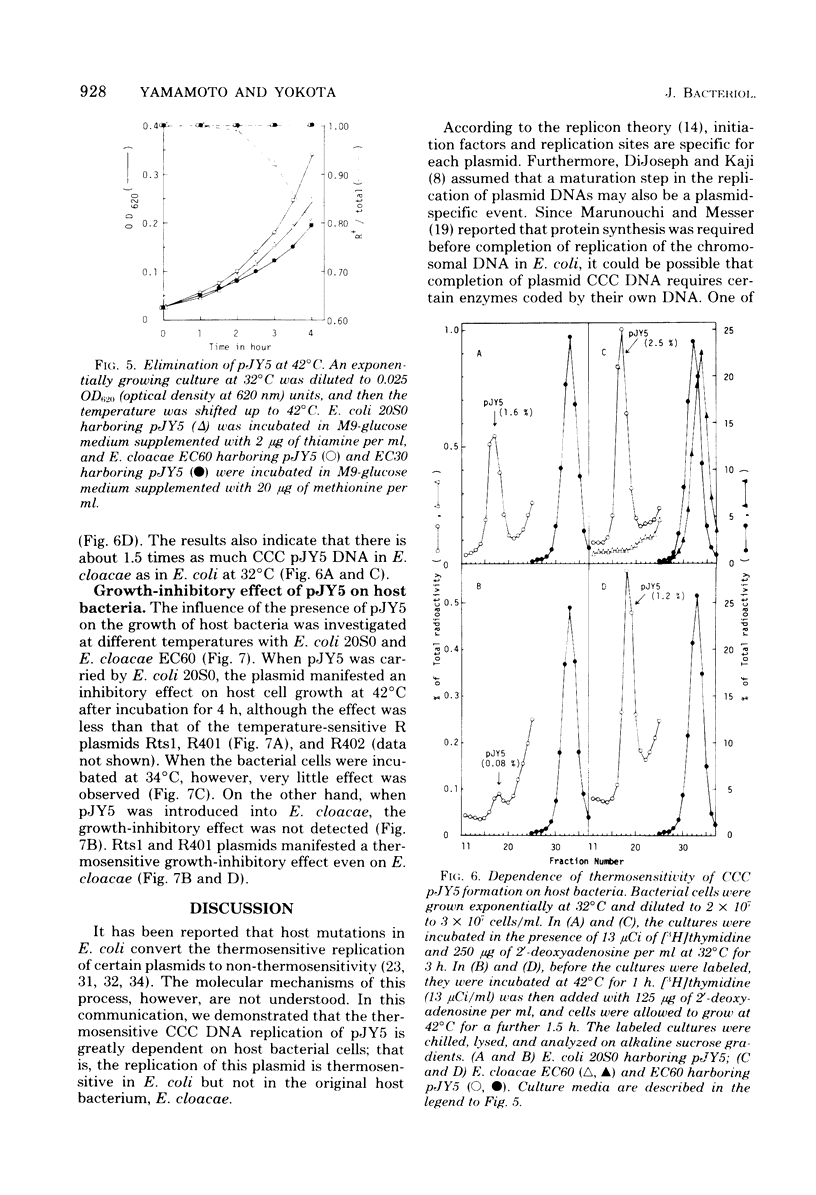
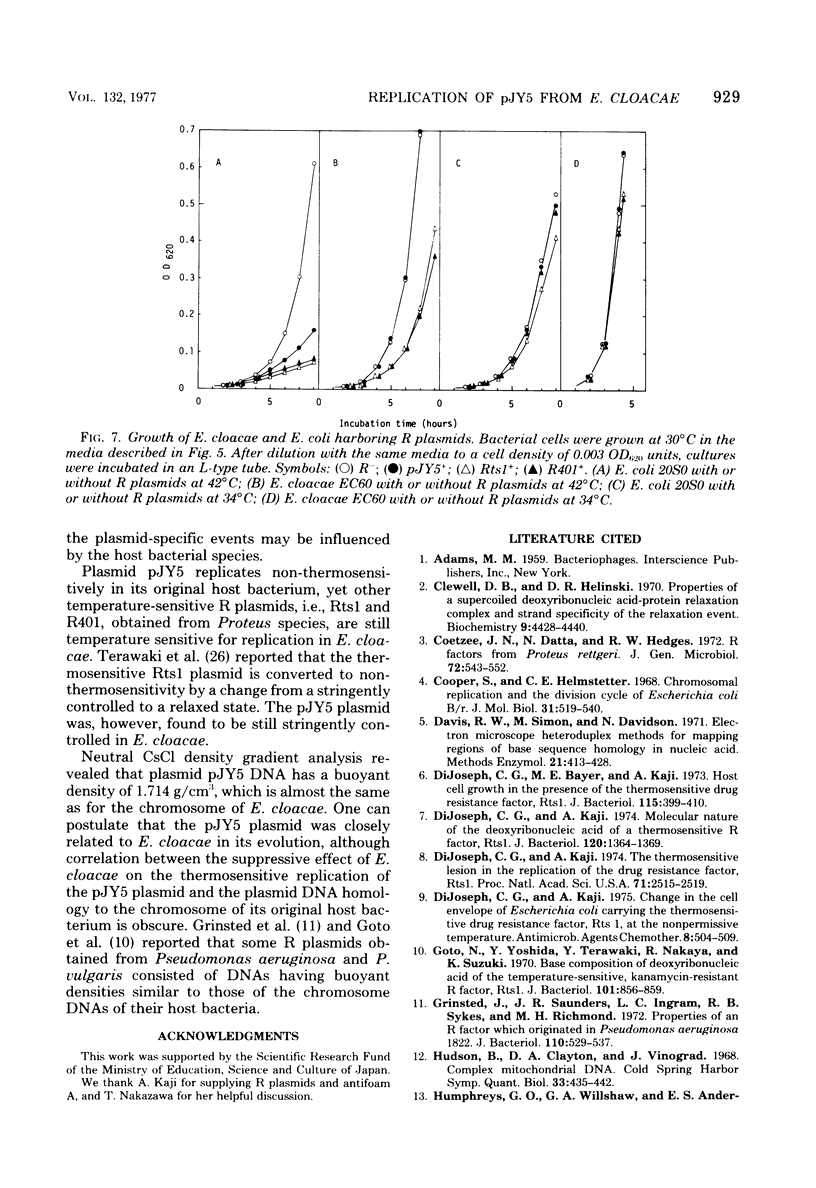
The thermosensitive replication of an R plasmid, pJY5, isolated from Enterobacter cloacae, was studied. pJY5 consisted of 61 million daltons of covalently closed circular (CCC) deoxyribonucleic acid (DNA) with a buoyant density of 1.714 g/cm3 (55 mol % guanine plus cytosine). In Escherichia coli, this plasmid replicated stringently at 32 degrees C, but ceased its CCC DNA replication after a short incubation at 42 degrees C, resulting in production of R- segregants. The thermosensitive replication of pJY5 was not overcome by the coexistence of non-thermosensitive R plasmids. The plasmid manifested an inhibitory effect on host bacterial cell growth at 42 degrees C, although the effect was less prominent than that of R plasmids belonging to the T-incompatibility group, Rts1, R401, and R402. When the pJY5 plasmid was transferred into E. cloacae, however, no R- segregants were detected at any culture temperature, even 42 degrees C. Alkaline sucrose gradient analysis revealed that a significant amount of pJY5 CCC DNA was synthesized in E. cloacae at the high temperature but not in E. coli. Furthermore, the growth-inhibitory effect of pJY5 on hosts at 42 degrees C was not observed in E. cloacae. On the other hand, Rts1 and R401 were found to be thermosensitive in E. cloacae as well as in E. coli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- DiJoseph C. G., Bayer M. E., Kaji A. Host cell growth in the presence of the thermosensitive drug resistance factor, Rts1. J Bacteriol. 1973 Jul;115(1):399–410. doi: 10.1128/jb.115.1.399-410.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G., Kaji A. Change in the cell envelope of Escherichia coli carrying the thermosensitive drug resistance factor, Rts 1, at the nonpermissive temperature. Antimicrob Agents Chemother. 1975 Oct;8(4):504–509. doi: 10.1128/aac.8.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G., Kaji A. Molecular nature of the deoxyribonucleic acid of a thermosensitive R factor, Rts1. J Bacteriol. 1974 Dec;120(3):1364–1369. doi: 10.1128/jb.120.3.1364-1369.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G. The thermosensitive lesion in the replication of the drug resistance factor, Rts1. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2515–2519. doi: 10.1073/pnas.71.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N., Yoshida Y., Terawaki Y., Nakaya R., Suzuki K. Base composition of deoxyribonucleic acid of the temperature-sensitive kanamycin-resistant R factor, Rts1. J Bacteriol. 1970 Mar;101(3):856–859. doi: 10.1128/jb.101.3.856-859.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Clayton D. A., Vinograd J. Complex mitochondrial DNA. Cold Spring Harb Symp Quant Biol. 1968;33:435–442. doi: 10.1101/sqb.1968.033.01.050. [DOI] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Marunouchi T., Messer W. Replication of a specific terminal chromosome segment in Escherichia coli which is required for cell division. J Mol Biol. 1973 Jun 25;78(1):211–228. doi: 10.1016/0022-2836(73)90439-7. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SUGINO Y., HIROTA Y. Conjugal fertility associated with resistance factor R in Escherichia coli. J Bacteriol. 1962 Nov;84:902–910. doi: 10.1128/jb.84.5.902-910.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Adelberg E. A. Temperature dependence of sex-factor maintenance in Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):447–449. doi: 10.1128/jb.109.1.447-449.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Ishizu K., Horiuchi S., Goto N., Nakaya R. Control of replication and segregation of R plasmid Rts1. J Bacteriol. 1976 Dec;128(3):693–700. doi: 10.1128/jb.128.3.693-700.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Rownd R. Replication of the R factor Rts1 in Proteus mirabilis. J Bacteriol. 1972 Feb;109(2):492–498. doi: 10.1128/jb.109.2.492-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Takayasu H., Akiba T. Thermosensitive replication of a kanamycin resistance factor. J Bacteriol. 1967 Sep;94(3):687–690. doi: 10.1128/jb.94.3.687-690.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoeda M., Inuzuka M., Anto S., Konishi M. Curing action of sodium dodecyl sulfate on a Proteus mirabilis R+ strain. J Bacteriol. 1974 Dec;120(3):1158–1163. doi: 10.1128/jb.120.3.1158-1163.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Spectinomycin resistance mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jun 28;67(3):533–535. doi: 10.1016/0022-2836(72)90472-x. [DOI] [PubMed] [Google Scholar]
- Yokota T., Kanamaru Y., Mori R., Akiba T. Recombination between a thermosensitive kanamycin resistance factor and a nonthermosensitive multiple-drug resistant factor. J Bacteriol. 1969 Jun;98(3):863–873. doi: 10.1128/jb.98.3.863-873.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]