Abstract
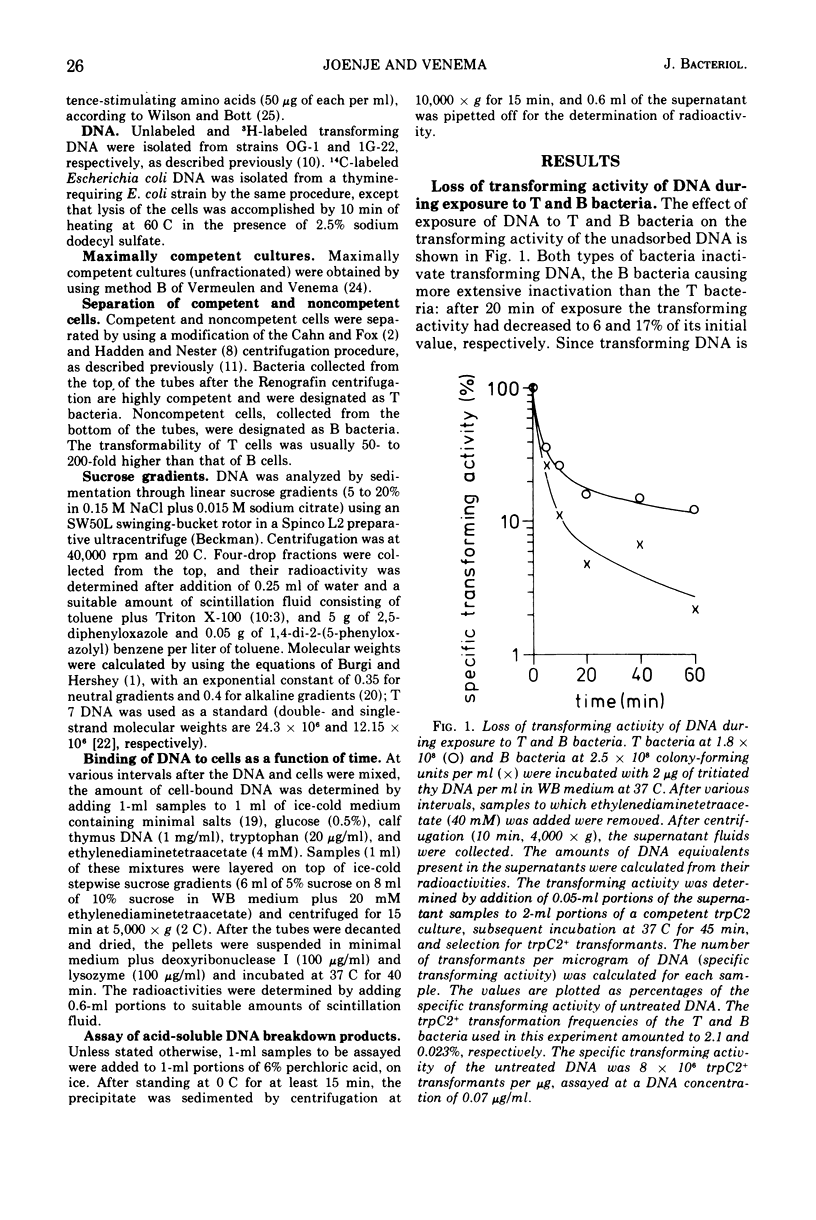
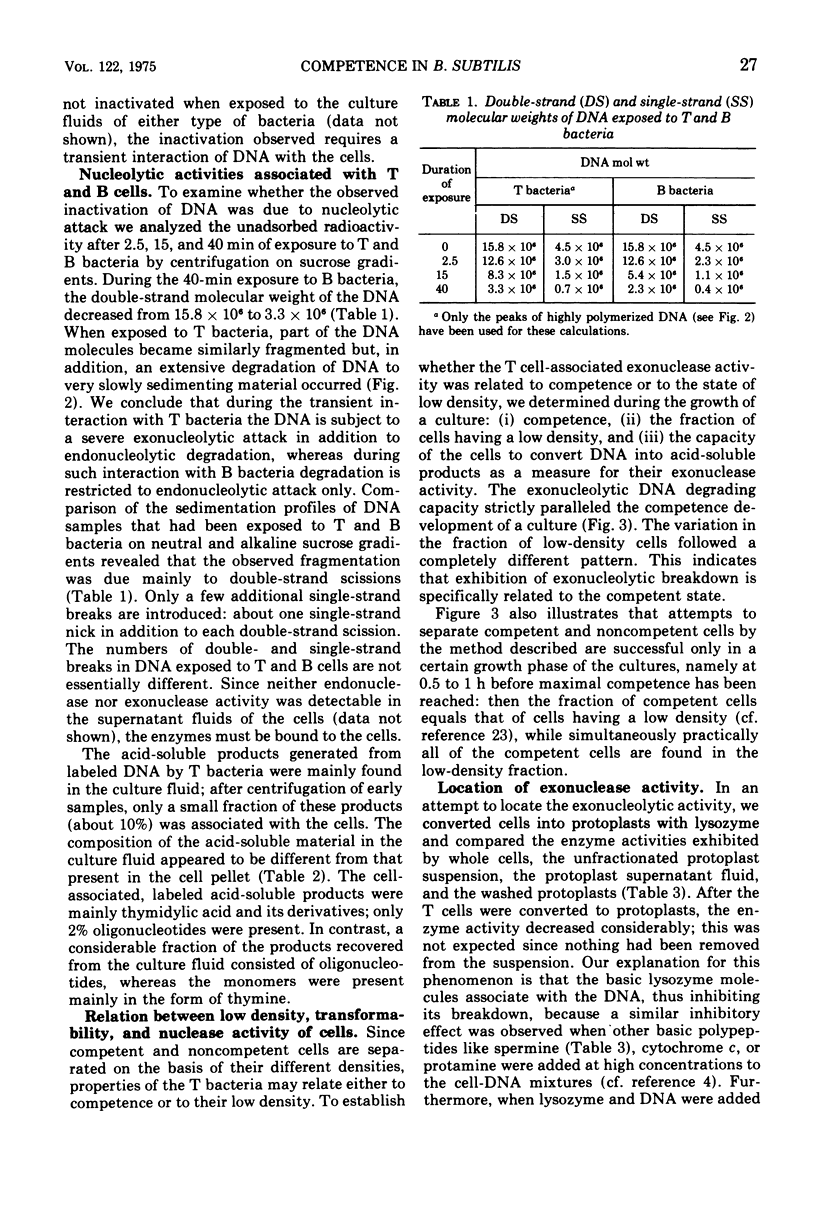
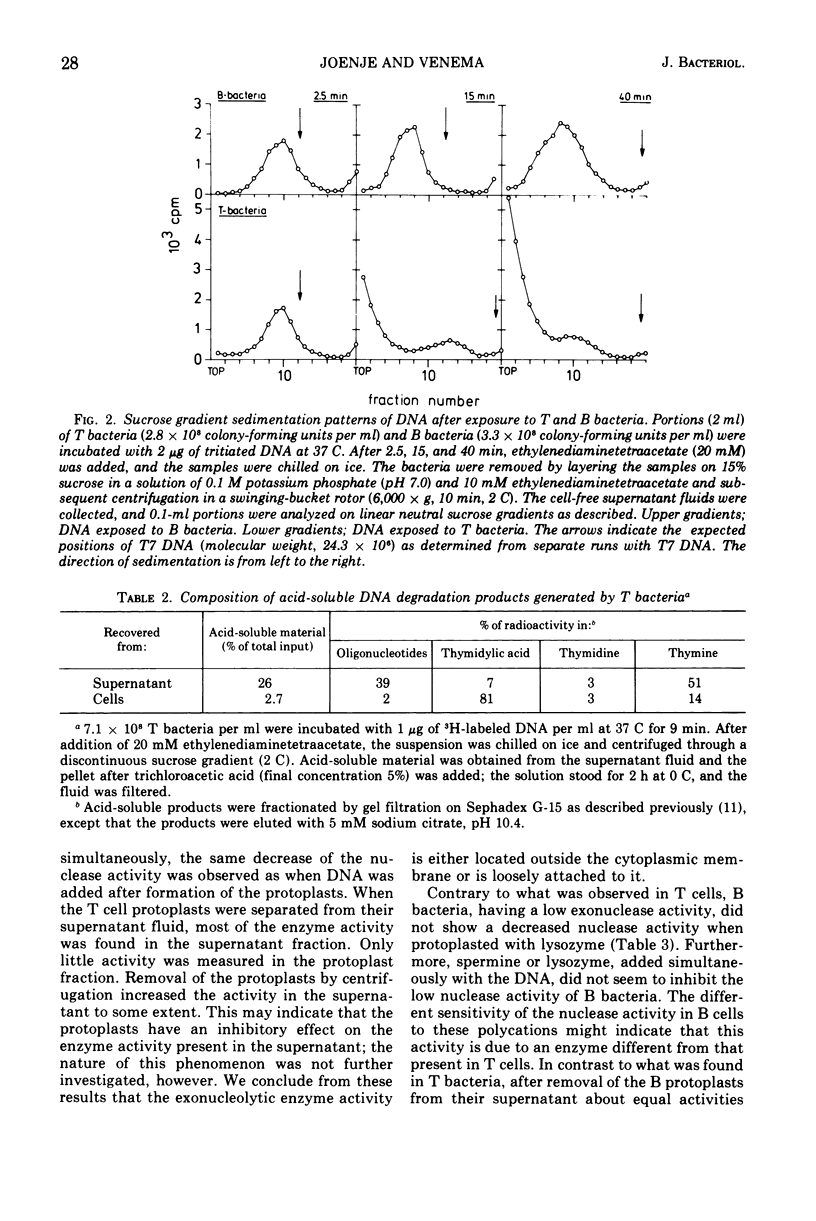
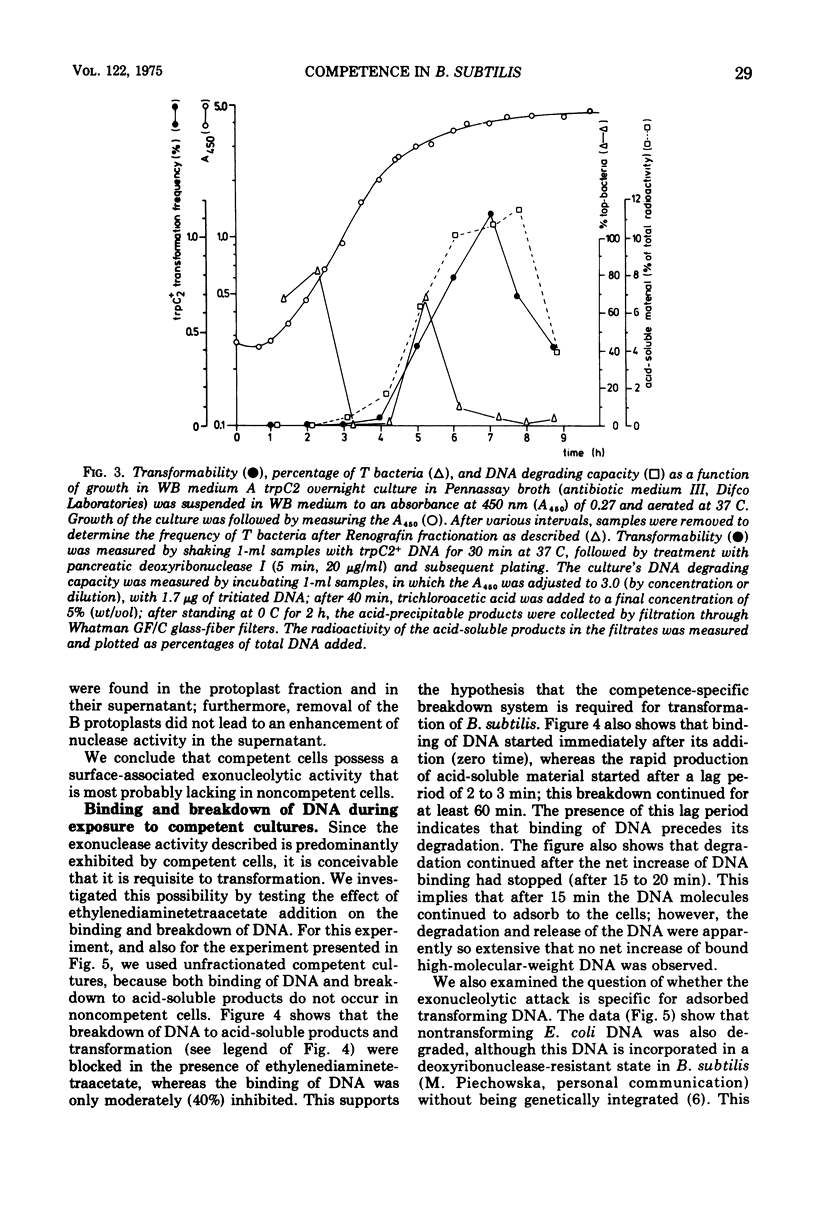
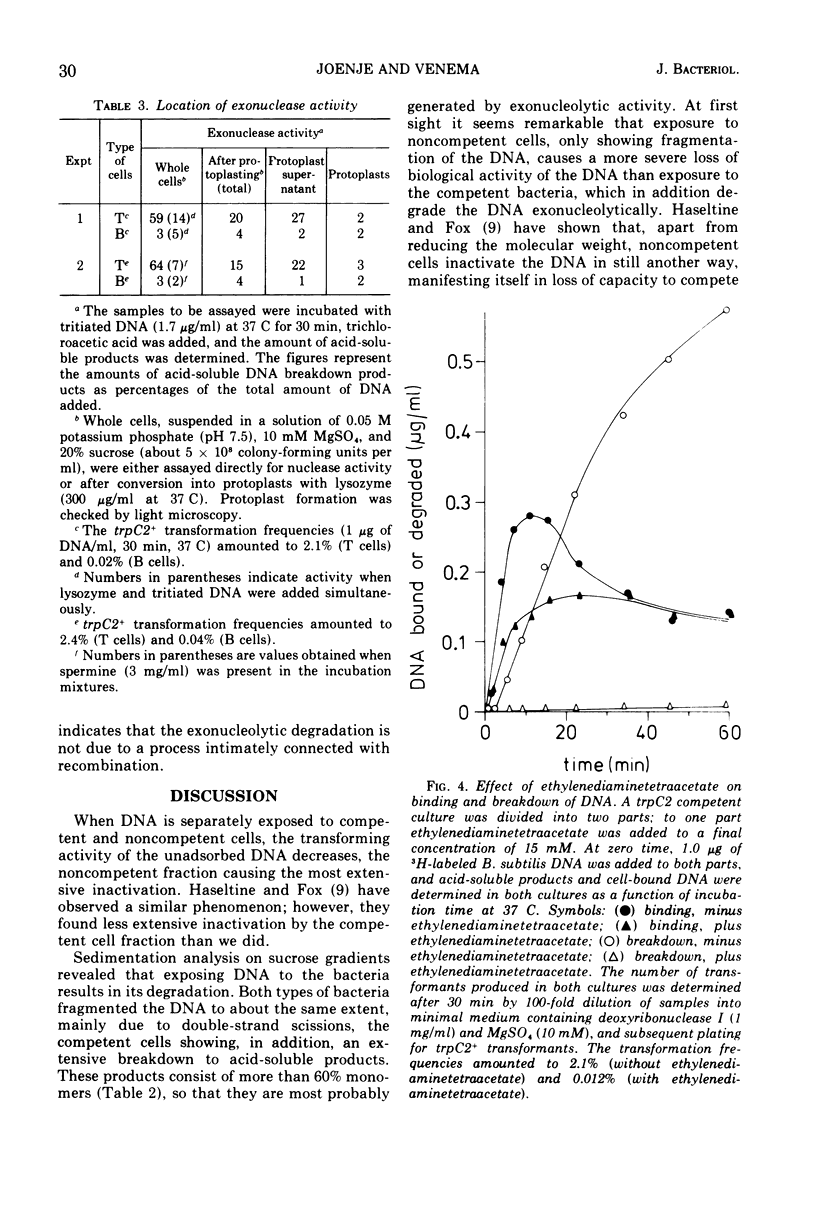
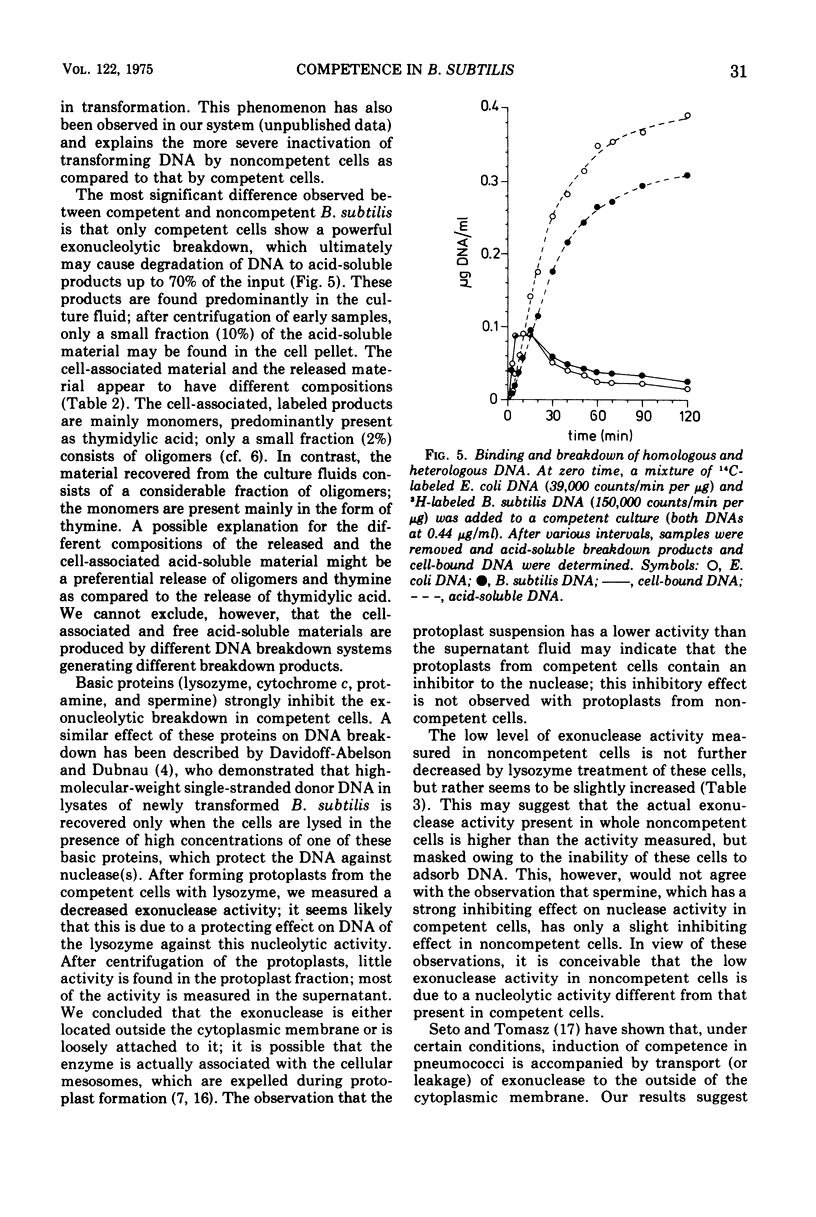
Competent and noncompetent cells of Bacillus subtilis were separated on the basis of their different buoyant densities. The two types of cells were compared with respect to their interactions with exogenous deoxyribonucleic acid(DNA). After exposure of DNA to the cells, the unadsorbed fraction of DNA molecules was examined. Both types of cells decreased the biological activity of this DNA, the inactiviation exerted by noncompetent cells being more severe than that exerted by competent cells. Sedimentation analysis of the inactivated DNA revealed that fragments of DNA are produced, owing mainly to the introduction of double-strand scissions. In addition to this fragmentation, the competent bacteria extensively digested the DNA exonucleolytically. This type of breakdown was specifically related to the competent state rather than to the state of low density. The exonucleolytic activity is, in all probability, associated with the cell envelope, because most of the activity is released into the medium when the cells are converted to protoplasts. At 37 C the competence-specific exonucleolytic breakdown started 2 to 3 min after the binding of DNA to the cells. In unfractionated cultures, breakdown may proceed until 70% of the total amount of DNA added has been made acid soluble. Nontransforming Escherichia coli DNA was also subject to exonucleolytic degradation; it seems unlikely,therefore, that this type of breakdown occurs as a consequence of recombination. Since ethylenediaminetetraacetate blocked both transformation by native DNA and the exonucleolytic breakdown of bound DNA, we suggest that the breakdown of DNA by competent cells fulfills an essential function in genetic transformation of B. subtilis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn F. H., Fox M. S. Fractionation of transformable bacteria from ocompetent cultures of Bacillus subtilis on renografin gradients. J Bacteriol. 1968 Mar;95(3):867–875. doi: 10.1128/jb.95.3.867-875.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Conditions affecting the isolation from transformed cells of Bacillus subtilis of high-molecular-weight single-stranded deoxyribonucleic acid of donor origin. J Bacteriol. 1973 Oct;116(1):146–153. doi: 10.1128/jb.116.1.146-153.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Kinetic analysis of the products of donor deoxyribonucleate in transformed cells of Bacillus subtilis. J Bacteriol. 1973 Oct;116(1):154–162. doi: 10.1128/jb.116.1.154-162.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. FATE OF THE MESOSOMES OF BACILLUS MEGATERIUM DURING PROTOPLASTING. J Bacteriol. 1964 Jun;87:1483–1491. doi: 10.1128/jb.87.6.1483-1491.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden C., Nester E. W. Purification of competent cells in the Bacillus subtilis transformation system. J Bacteriol. 1968 Mar;95(3):876–885. doi: 10.1128/jb.95.3.876-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine F. P., Fox M. S. Bacterial inactivation of transforming deoxyribonucleate. J Bacteriol. 1971 Sep;107(3):889–899. doi: 10.1128/jb.107.3.889-899.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joenje H., Konings W. N., Venema G. Interactions between exogenous deoxyribonucleic acid and membrane vesicles isolated from Bacillus subtilis 168. J Bacteriol. 1974 Sep;119(3):784–794. doi: 10.1128/jb.119.3.784-794.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joenje H., Konings W. N., Venema G. Interactions between exogenous deoxyribonucleic acid and membrane vesicles isolated from competent and noncompetent Bacillus subtilis. J Bacteriol. 1975 Mar;121(3):771–776. doi: 10.1128/jb.121.3.771-776.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Role of a deoxyribonuclease in the genetic transformation of Diplococcus pneumoniae. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2305–2309. doi: 10.1073/pnas.71.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Early intermediate state of transforming deoxyribonucleic acid during uptake by Bacillus subtilis. J Bacteriol. 1971 Oct;108(1):38–44. doi: 10.1128/jb.108.1.38-44.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechowska M., Fox M. S. Fate of transforming deoxyribonucleate in Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):680–689. doi: 10.1128/jb.108.2.680-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Frehel C., Ferrandes B. Comportement des mésosomes lors de l'attaque de Bacillus subtilis par le lysozyme en milieu hyper- ou hypotonique. C R Acad Sci Hebd Seances Acad Sci D. 1967 Oct 23;265(17):1259–1262. [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Seto H., Tomasz A. Early stages in DNA binding and uptake during genetic transformation of pneumococci. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1493–1498. doi: 10.1073/pnas.71.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Tomasz A. Protoplast formation and leakage of intramembrane cell components: induction by the competence activator substance of pneumococci. J Bacteriol. 1975 Jan;121(1):344–353. doi: 10.1128/jb.121.1.344-353.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia M. J., Mandel M. Nature of the ethylenediaminetetraacetic acid requirement for transformation of Bacillus subtilis with single-stranded deoxyribonucleic acid. J Bacteriol. 1970 Mar;101(3):844–850. doi: 10.1128/jb.101.3.844-850.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen C. A., Venema G. Autoradiographic estimation of competence and the relationship between competence and transformability in cultures of Bacillus subtilis. J Gen Microbiol. 1971 Dec;69(2):239–252. doi: 10.1099/00221287-69-2-239. [DOI] [PubMed] [Google Scholar]
- Vermeulen C. A., Venema G. The effect of competence regime on competence, DNA absorption and integration of DNA in cultures of Bacillus subtilis. J Gen Microbiol. 1972 Aug;71(3):415–424. doi: 10.1099/00221287-71-3-415. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Bott K. F. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968 Apr;95(4):1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


