Abstract
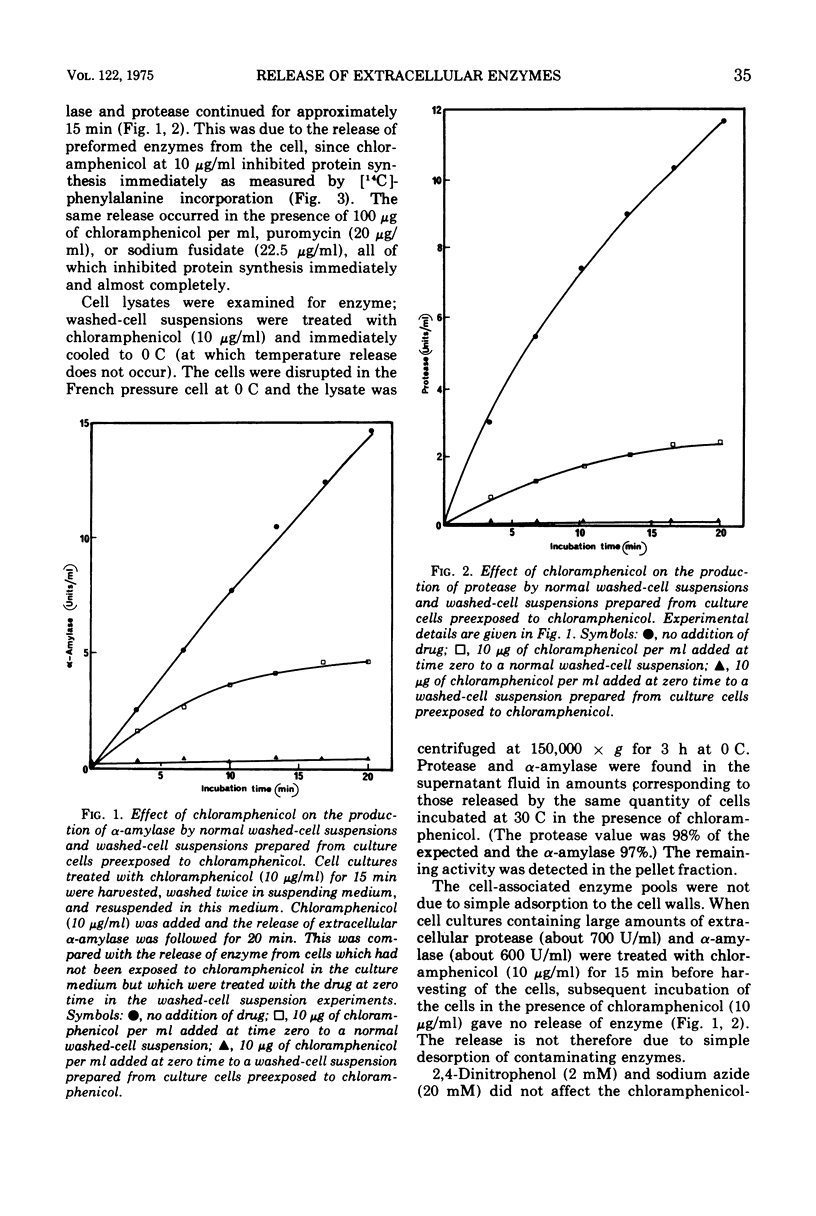
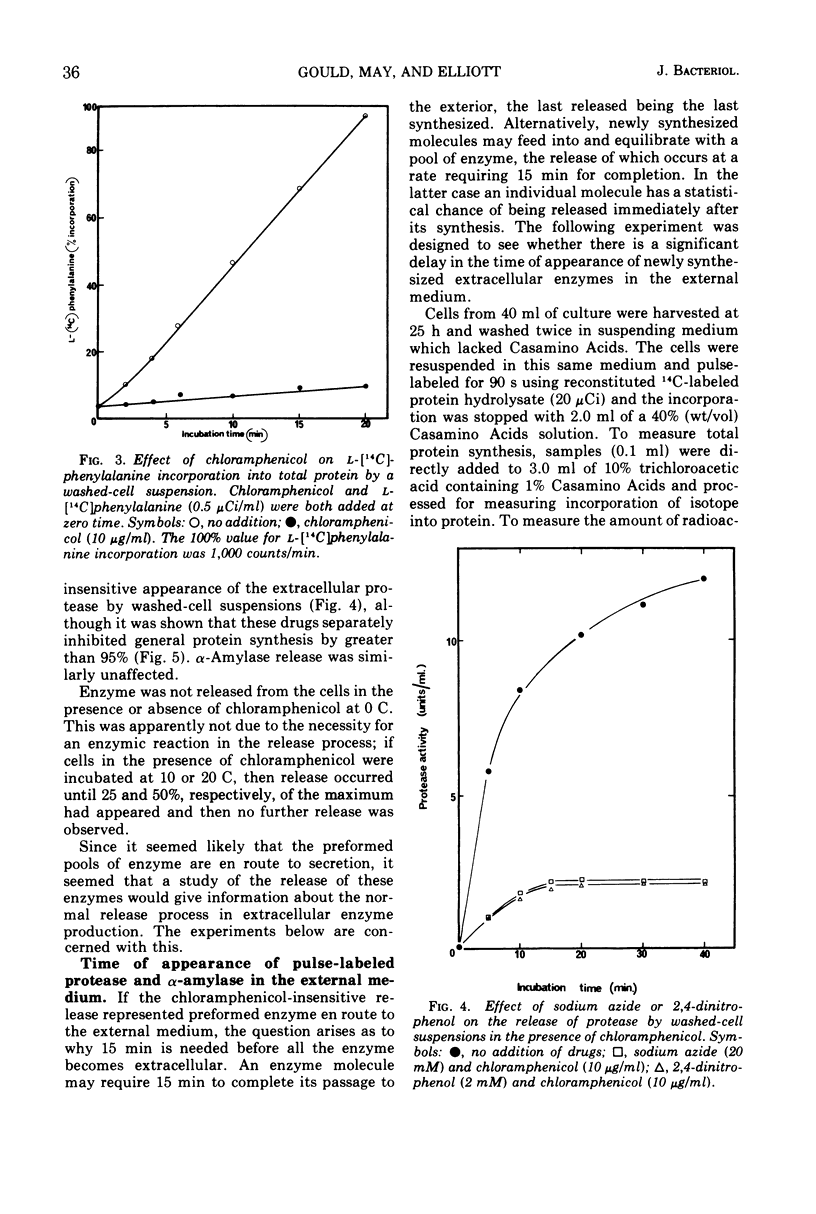
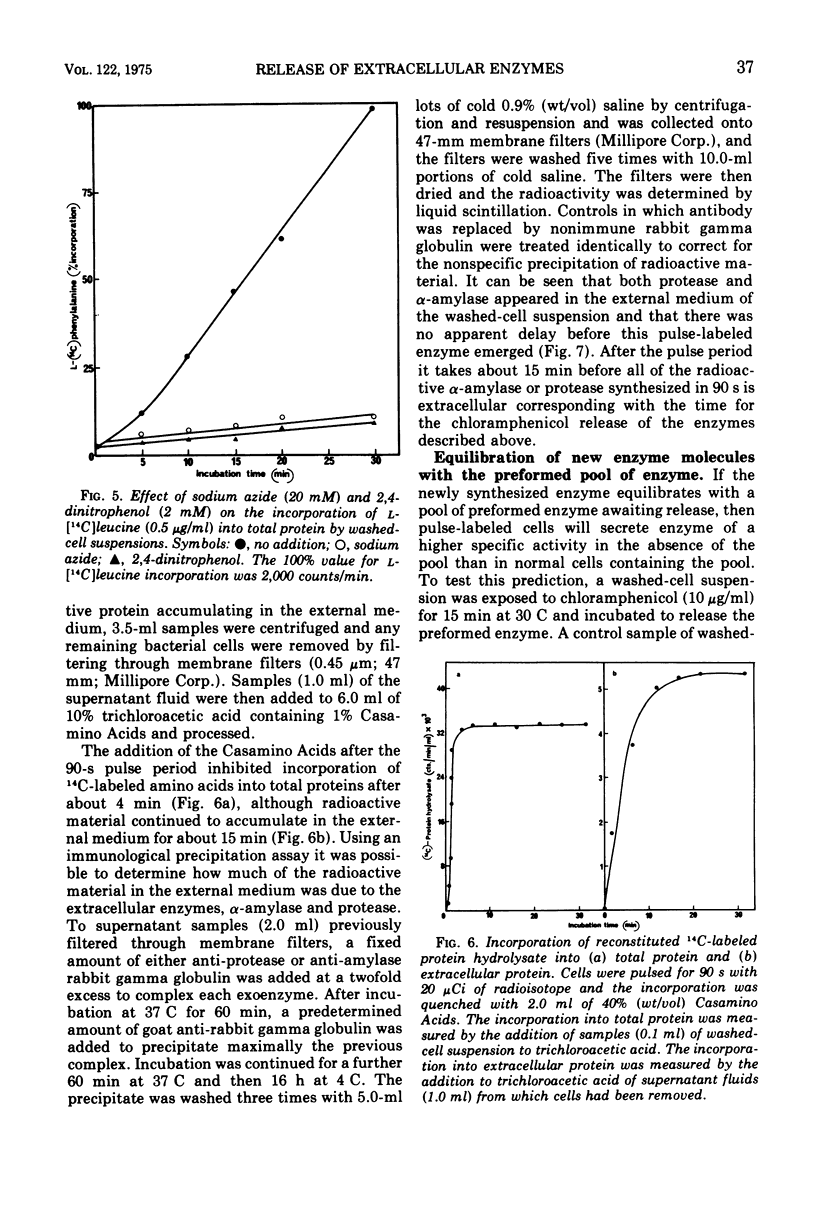
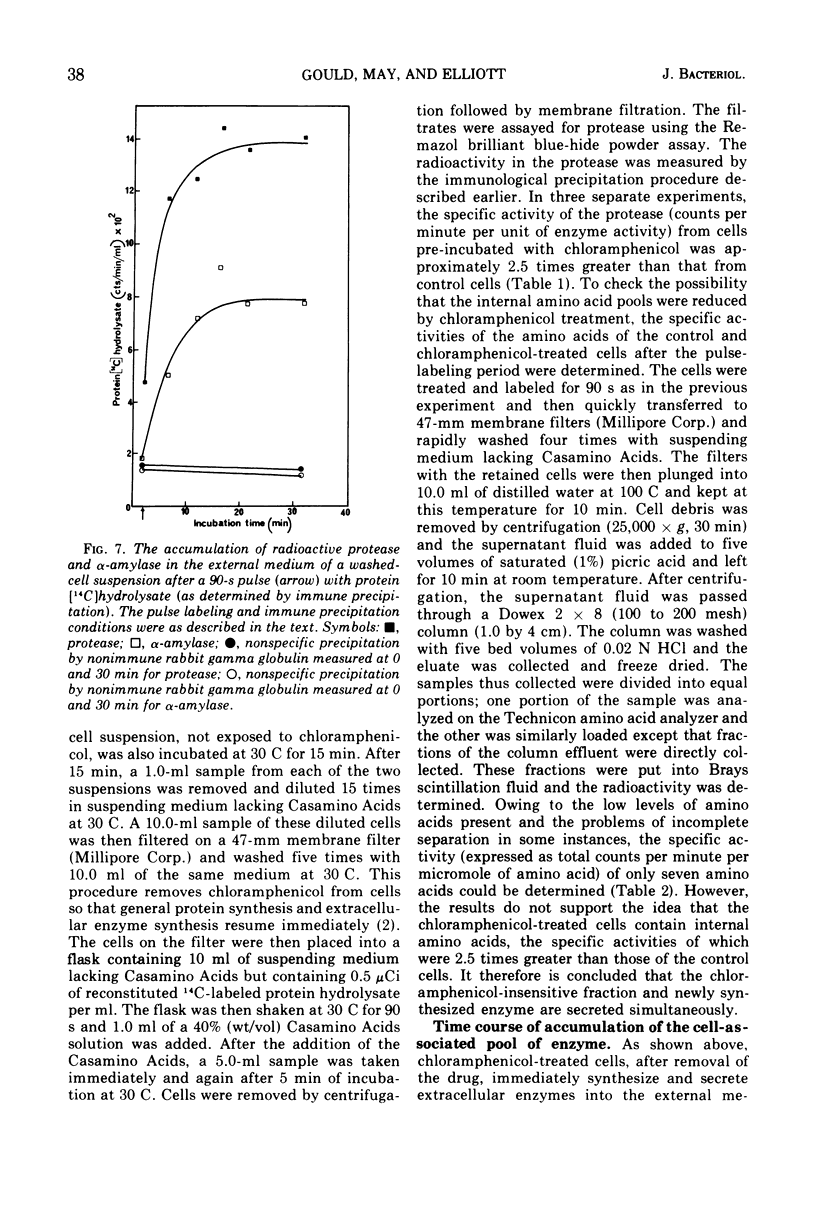
Washed-cell suspensions of Bacillus amyloliquefaciens secrete significant amounts of the extracellular enzymes alpha-amylase and protease for about 15 min in the almost complete absence of protein synthesis. This apparently represents release of preformed enzyme en route to secretion. The release was independent of energy but was affected by temperature. Pulse-labeling experiments showed that newly synthesized enzyme molecules are either immediately released into the external medium or equilibrate with the preformed enzyme prior to eventual secretion. The results are compatible with a model of secretion whereby enzyme molecules emerging from the cell membrane become temporarily restricted by the cell wall so that a small pool of active enzyme accumulates in this region.
Full text
PDF
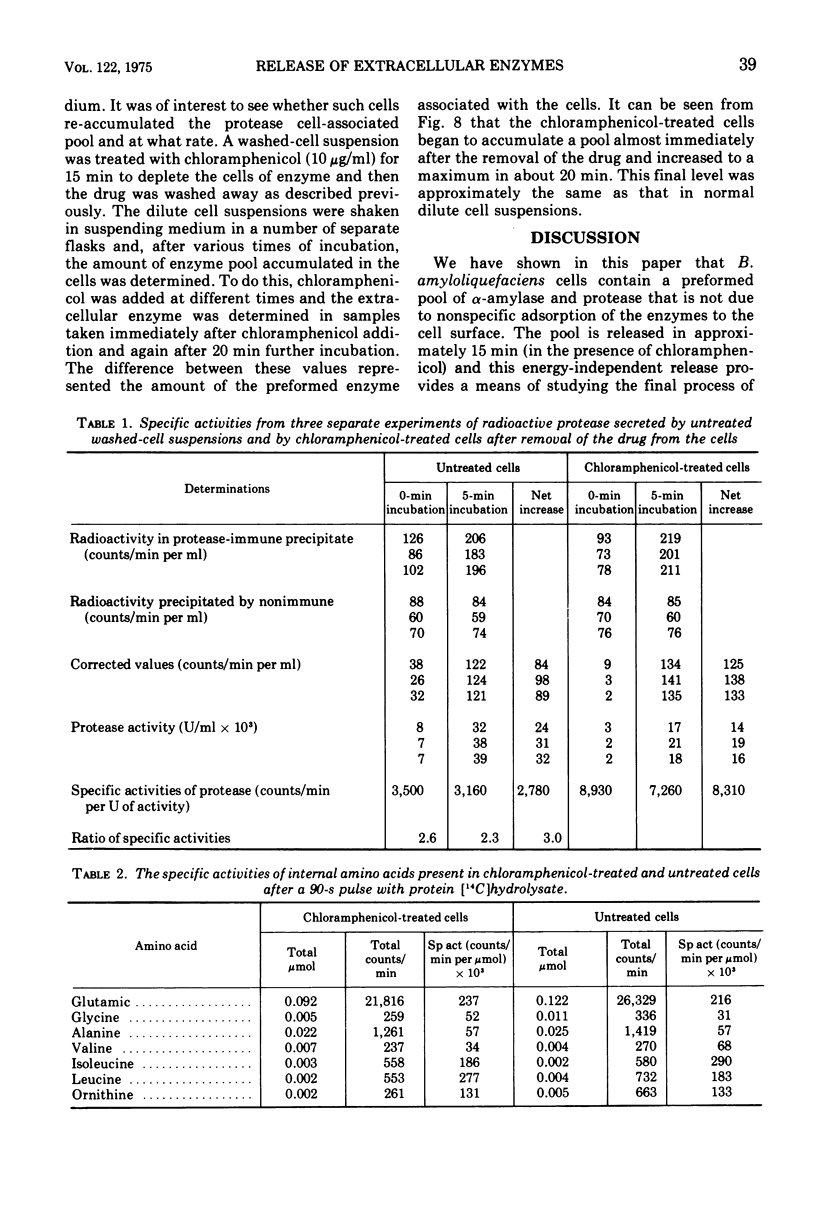
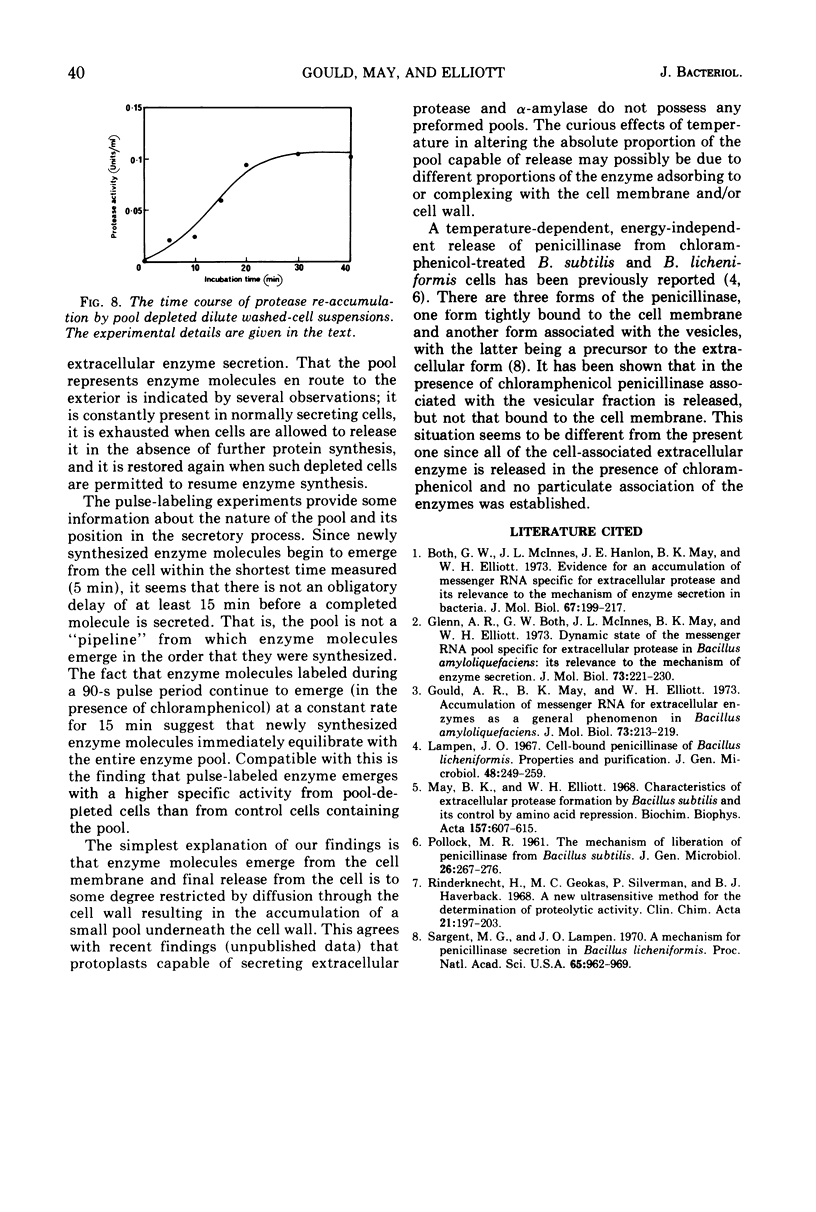
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Both G. W., McInnes J. L., Hanlon J. E., May B. K., Elliott W. H. Evidence for an accumulation of messenger RNA specific for extracellular protease and its relevance to the mechanism of enzyme secretion in bacteria. J Mol Biol. 1972 Jun 20;67(2):199–217. doi: 10.1016/0022-2836(72)90236-7. [DOI] [PubMed] [Google Scholar]
- Glenn A. R., Both G. W., McInnes J. L., May B. K., Elliott W. H. Dynamic state of the messenger RNA pool specific for extracellular protease in Bacillus amyloliquefaciens: its relevance to the mechanism of enzyme secretion. J Mol Biol. 1973 Jan 10;73(2):221–230. doi: 10.1016/0022-2836(73)90325-2. [DOI] [PubMed] [Google Scholar]
- Gould A. R., May B. K., Elliott W. H. Accumulation of messenger RNA for extracellular enzymes as a general phenomenon in Bacillus amyloiquefaciens. J Mol Biol. 1973 Jan 10;73(2):213–219. doi: 10.1016/0022-2836(73)90324-0. [DOI] [PubMed] [Google Scholar]
- Lampen J. O. Cell-bound penicillinase of Bacillus licheniformis; properties and purification. J Gen Microbiol. 1967 Aug;48(2):249–259. doi: 10.1099/00221287-48-2-249. [DOI] [PubMed] [Google Scholar]
- May B. K., Elliott W. H. Characteristics of extracellular protease formation by Bacillus subtilis and its control by amino acid repression. Biochim Biophys Acta. 1968 May 21;157(3):607–615. doi: 10.1016/0005-2787(68)90158-5. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. The mechanism of liberation of penicillinase from Bacillus subtilis. J Gen Microbiol. 1961 Oct;26:267–276. doi: 10.1099/00221287-26-2-267. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Silverman P., Haverback B. J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- Sargent M. G., Lampen J. O. A mechanism for penicillinasesecretion in Bacillus licheniformis. Proc Natl Acad Sci U S A. 1970 Apr;65(4):962–969. doi: 10.1073/pnas.65.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]


