Abstract
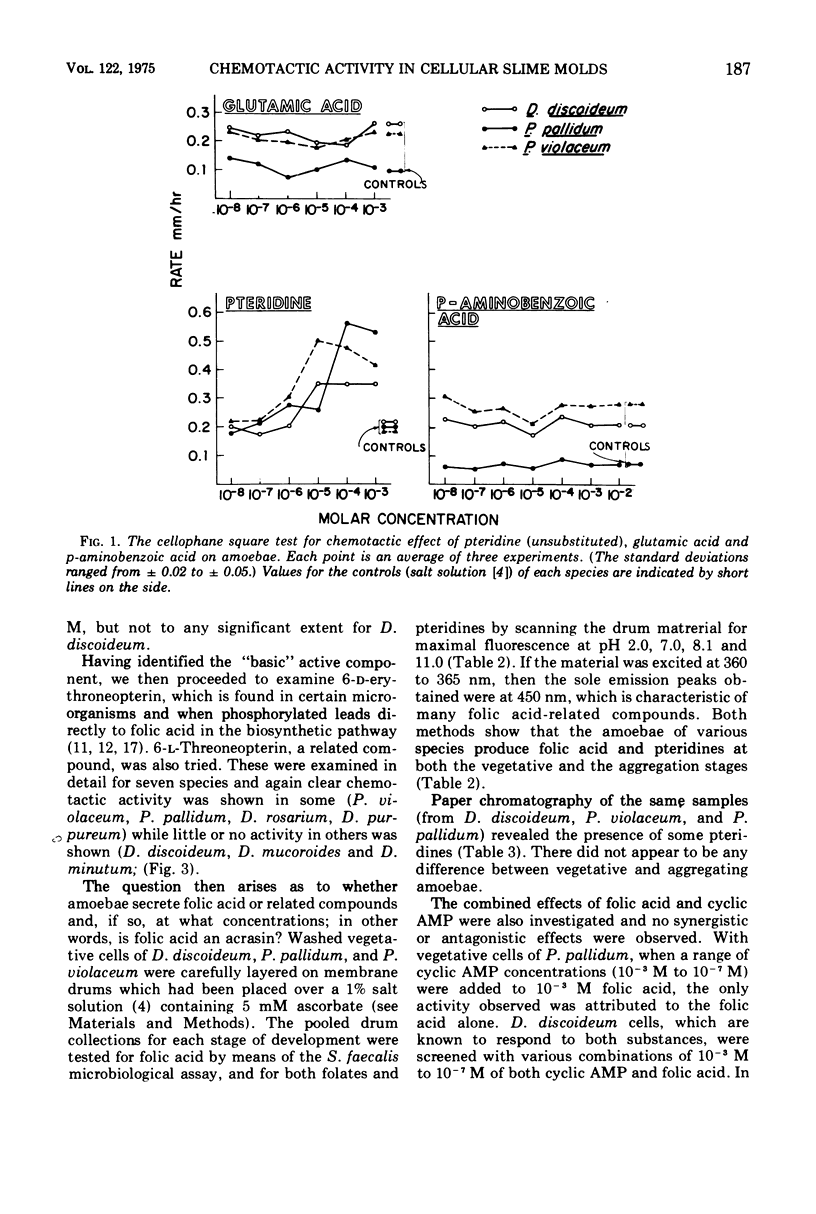
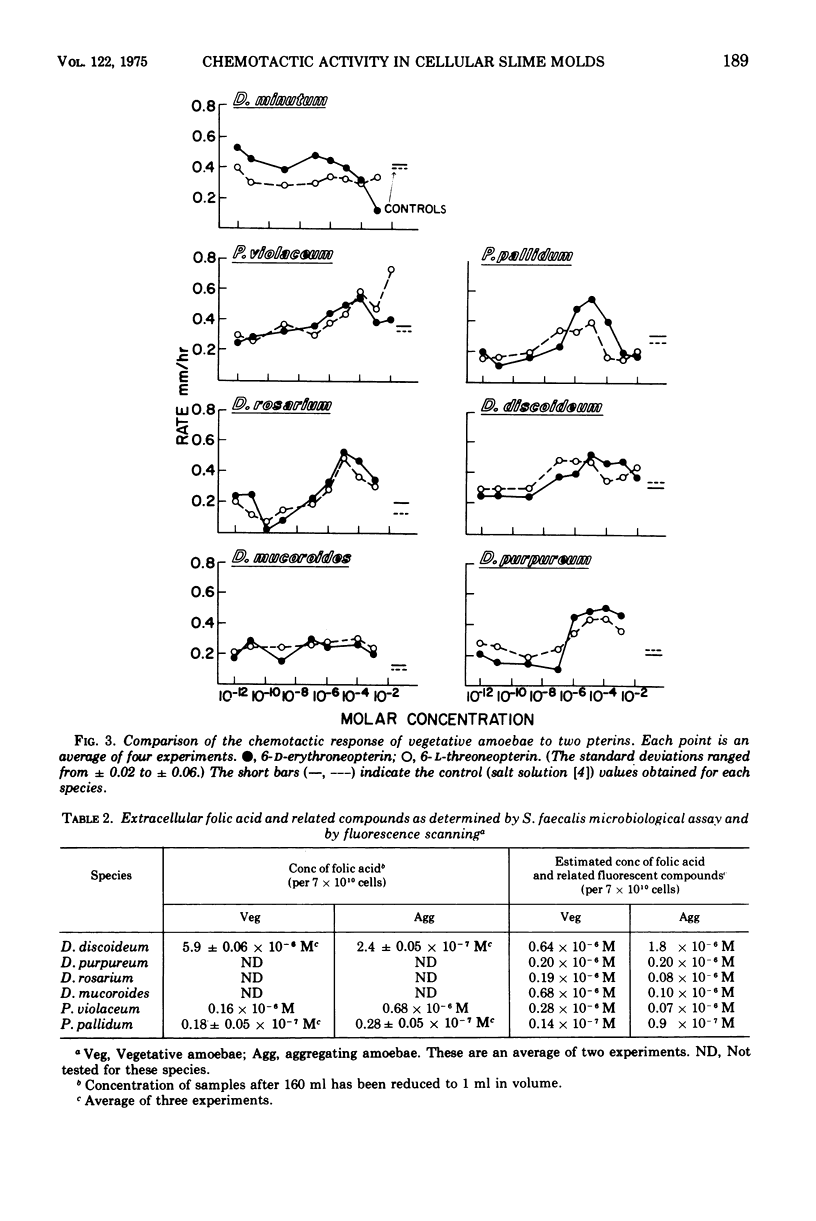
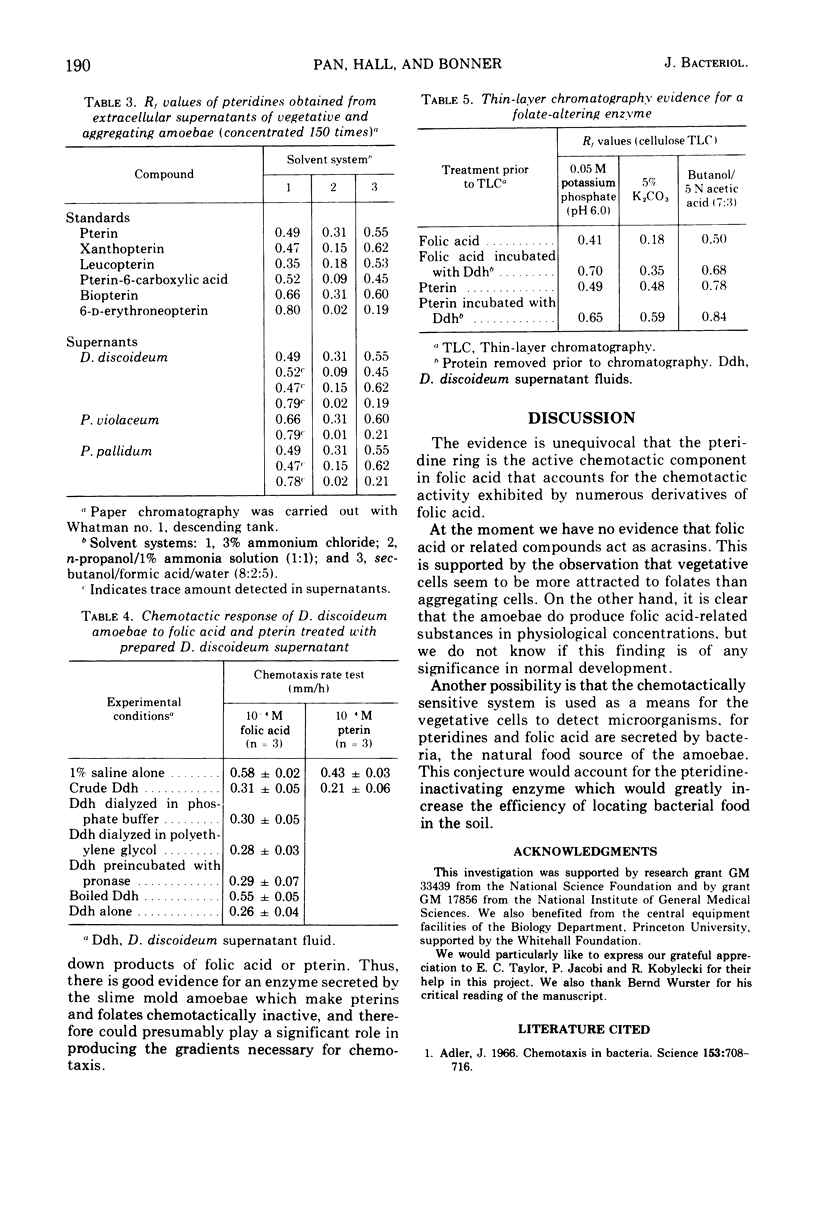
From earlier work it is known that folic acid attracts the amoebae of various species of cellular slime molds (11). Here we have tested a wide variety of pteridines, pyrimidines, and pyrazines to determine what part of the folic acid molecule is chemotactically active. It was shown that the activity lies in the pteridine ring itself. Furthermore, the cell-free supernatants of slime mold amoebae contain an enzyme that renders pterin and folic acid chemotactically inactive, which apparently increases the chemotactic sensitivity of the amoebae to those compounds. Despite the fact that slime mold amoebae secrete small amounts of folic acid-related compounds, there is no evidence that folates are acrasins; rather it is postulated that attraction to folates may be a food-seeking device for the amoebae which prey on folate-secreting bacteria in the soil.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. T. Aggregation and differentiation in the cellular slime molds. Annu Rev Microbiol. 1971;25:75–92. doi: 10.1146/annurev.mi.25.100171.000451. [DOI] [PubMed] [Google Scholar]
- Bonner J. T., Barkley D. S., Hall E. M., Konijn T. M., Mason J. W., O'Keefe G., 3rd, Wolfe P. B. Acrasin, Acrasinase, and the sensitivity to acrasin in Dictyostelium discoideum. Dev Biol. 1969 Jul;20(1):72–87. doi: 10.1016/0012-1606(69)90005-0. [DOI] [PubMed] [Google Scholar]
- Bonner J. T., Kelso A. P., Gillmor R. G. A new approach to the problem of aggregation in the cellular slime molds. Biol Bull. 1966 Feb;130(1):28–42. doi: 10.2307/1539950. [DOI] [PubMed] [Google Scholar]
- Gerisch G. Cell aggregation and differentiation in Dictyostelium. Curr Top Dev Biol. 1968;3:157–197. doi: 10.1016/s0070-2153(08)60354-3. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Shiota T. Identification of the isomer of dihydroneopterin triphosphate synthesized by two enzyme fractions from Lactobacillus plantarum. J Biol Chem. 1971 Dec 25;246(24):7454–7459. [PubMed] [Google Scholar]
- Jones T. H., Brown G. M. The biosynthesis of folic acid. VII. Enzymatic synthesis of pteridines from guanosine triphosphate. J Biol Chem. 1967 Sep 25;242(18):3989–3997. [PubMed] [Google Scholar]
- Pan P., Hall E. M., Bonner J. T. Folic acid as second chemotactic substance in the cellular slime moulds. Nat New Biol. 1972 Jun 7;237(75):181–182. doi: 10.1038/newbio237181a0. [DOI] [PubMed] [Google Scholar]
- REYNOLDS J. J., BROWN G. M. THE BIOSYNTHESIS OF FOLIC ACID. IV. ENZYMATIC SYNTHESIS OF DIHYDROFOLIC ACID FROM GUANINE AND RIBOSE COMPOUNDS. J Biol Chem. 1964 Jan;239:317–325. [PubMed] [Google Scholar]
- ROBERTS D. Cleavage of folic acid by hydrogen peroxide. Biochim Biophys Acta. 1961 Dec 23;54:572–574. doi: 10.1016/0006-3002(61)90097-x. [DOI] [PubMed] [Google Scholar]



