Abstract
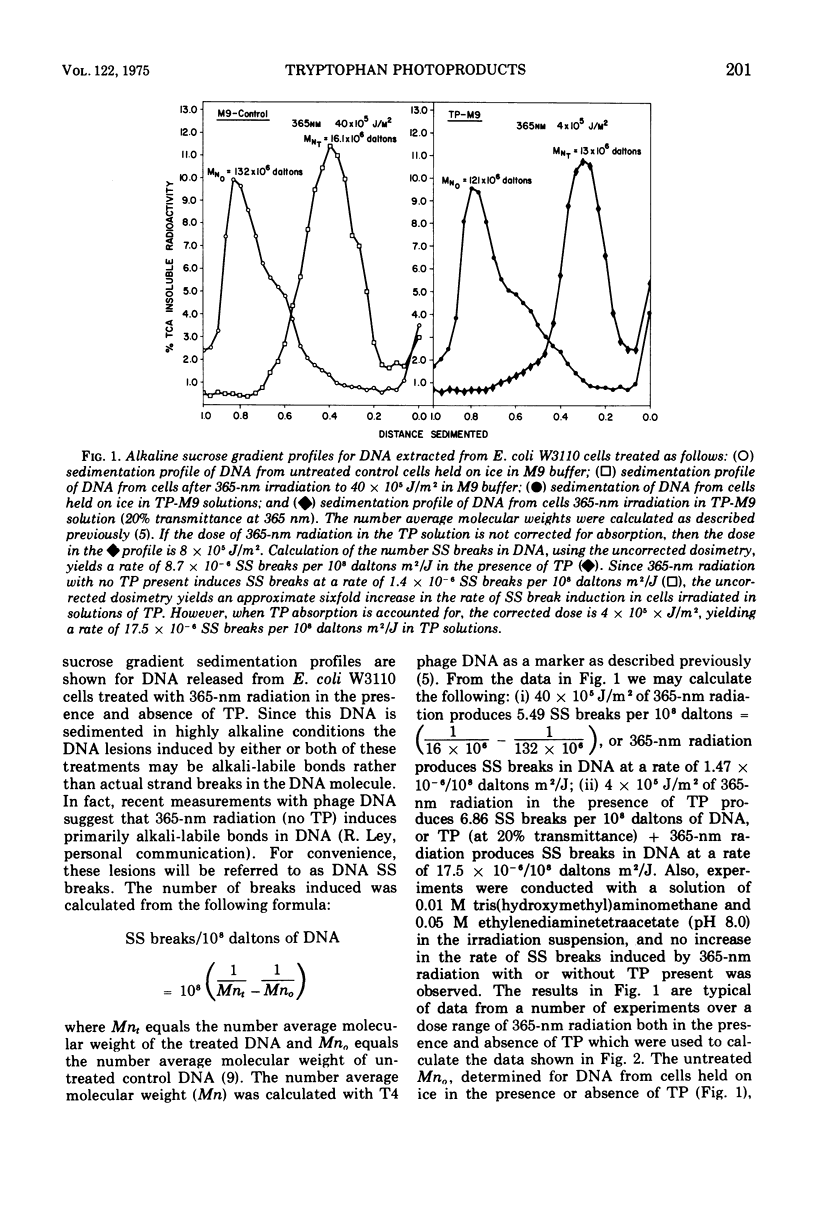
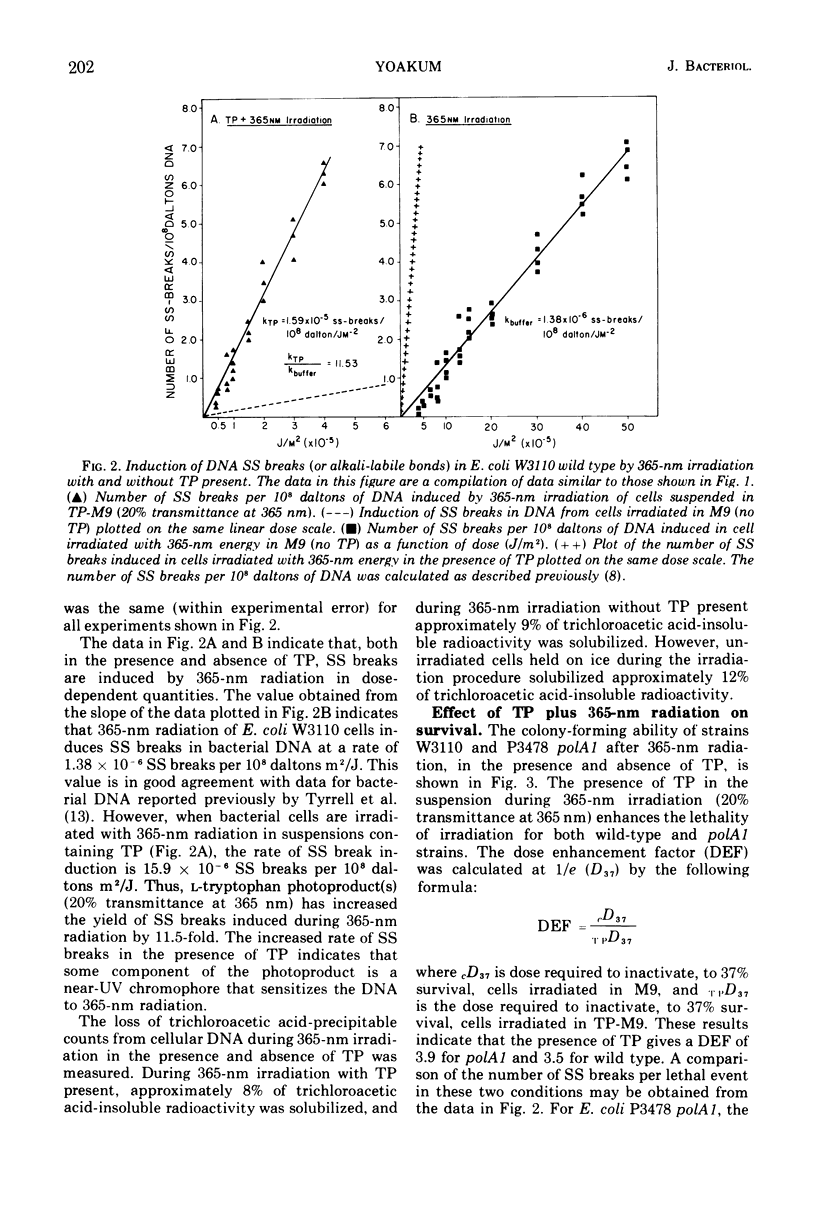
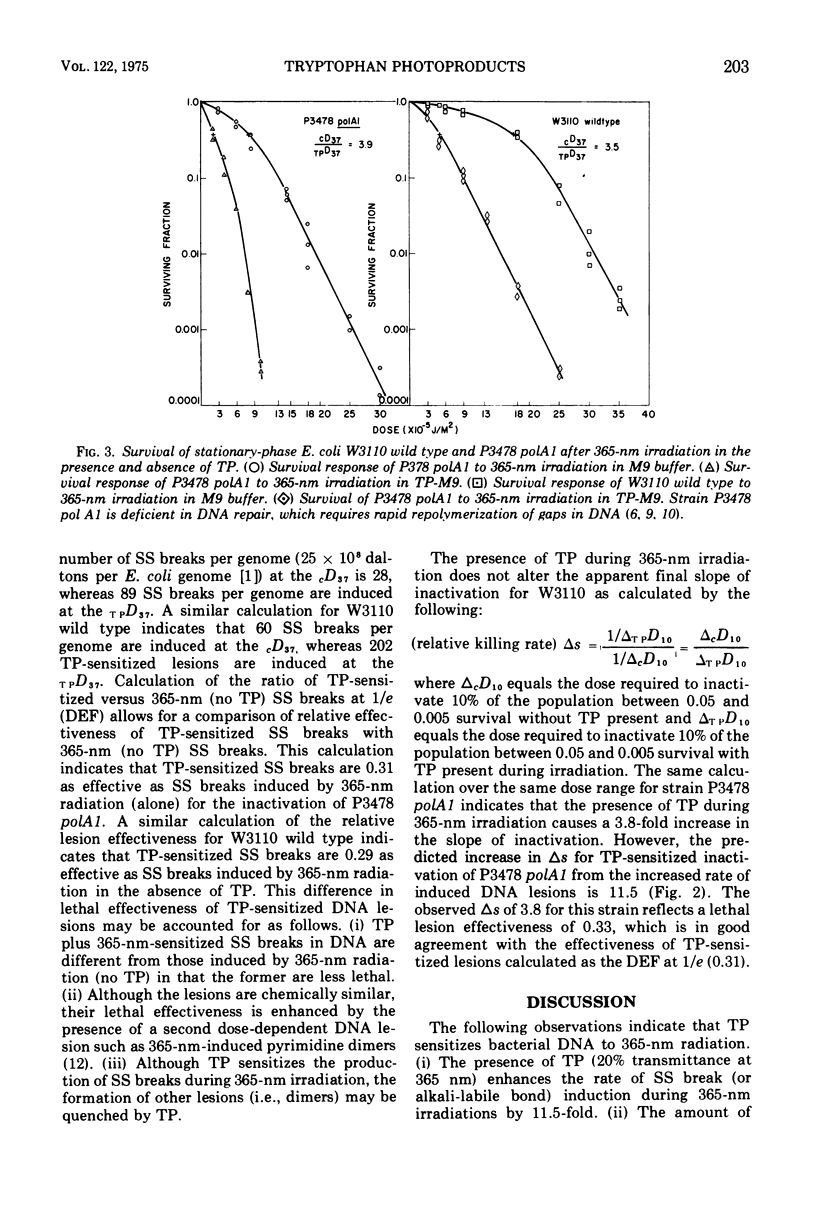
Long-wavelength ultraviolet light (300 to 400 nm) converts L-tryptophan to a photoproduct that is toxic for bacterial cells in dark conditions. We now report that similar photoproducts of l-tryptophan sensitize bacterial deoxyribonucleic acid to 365-nm radiation, increasing the yield of deoxyribonucleic acid strand breaks (or alkali-labile bonds) by approximately 11.5-fold. Evidence is also presented which indicates that thse sensitized deoxyribonucleic acid lesions contribute to lethality for Escherichia coli irradiated with 365-nm ultraviolet light in suspensions of tryptophan photoproducts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- Kanner L., Hanawalt P. Repair deficiency in a bacterial mutant defective in DNA polymerase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):149–155. doi: 10.1016/0006-291x(70)90770-9. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E. Mutagenesis by near-visible light. Science. 1967 Mar 24;155(3769):1545–1546. doi: 10.1126/science.155.3769.1545. [DOI] [PubMed] [Google Scholar]
- Ley R. D. Postreplication repair in an excision-defective mutant of Escherichia coli: ultraviolet light-induced incorporation of bromodeoxyuridine into parental DNA. Photochem Photobiol. 1973 Aug;18(2):87–95. doi: 10.1111/j.1751-1097.1973.tb06397.x. [DOI] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Setlow J. K. Effects of radiation on polynucleotides. Annu Rev Biophys Bioeng. 1972;1:293–346. doi: 10.1146/annurev.bb.01.060172.001453. [DOI] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. DNA polymerase required for rapid repair of x-ray--induced DNA strand breaks in vivo. Science. 1971 May 21;172(3985):851–854. doi: 10.1126/science.172.3985.851. [DOI] [PubMed] [Google Scholar]
- Town C. D., Smith K. C., Kaplan H. S. The rapair of DNA single-strand breaks in E. coli K-12 x-irradiated in the presence or absence of oxygen; the influence of repair on cell survival. Radiat Res. 1973 Aug;55(2):334–345. [PubMed] [Google Scholar]
- Tyrrell R. M. Induction of pyrimidine dimers in bacterial DNA by 365 nm radiation. Photochem Photobiol. 1973 Jan;17(1):69–73. doi: 10.1111/j.1751-1097.1973.tb06334.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M., Ley R. D., Webb R. B. Induction of single-strand breaks (alkali-labile bonds) in bacterial and phage DNA by near UV (365 nm) radiation. Photochem Photobiol. 1974 Nov;20(5):395–398. doi: 10.1111/j.1751-1097.1974.tb06593.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M. The interaction of near U.V. (365nm) and x-radiations on wild-type and repair-deficient strains of Escherichia coli K12: physical and biological measurements. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Apr;25(4):373–390. doi: 10.1080/09553007414550441. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M., Webb R. B. Reduced dimer excision in bacteria following near ultraviolet (365 nm) radiation. Mutat Res. 1973 Sep;19(3):361–364. doi: 10.1016/0027-5107(73)90238-8. [DOI] [PubMed] [Google Scholar]
- Wang R. J., Stoien J. D., Landa F. Lethal effect of near-ultraviolet irradiation on mammalian cells in culture. Nature. 1974 Jan 4;247(5435):43–45. doi: 10.1038/247043a0. [DOI] [PubMed] [Google Scholar]
- Webb R. B., Lorenz J. R. Oxygen dependence and repair of lethal effects of near ultraviolet and visible light. Photochem Photobiol. 1970 Oct;12(4):283–289. doi: 10.1111/j.1751-1097.1970.tb06060.x. [DOI] [PubMed] [Google Scholar]
- Webb R. B., Malina M. M. Mutagenesis in Escherichia coli by visible light. Science. 1967 May 26;156(3778):1104–1105. doi: 10.1126/science.156.3778.1104. [DOI] [PubMed] [Google Scholar]
- Webb R. B., Malina M. M. Mutagenic effects of near ultraviolet and visible radiant energy on continuous cultures of escherichia coli. Photochem Photobiol. 1970 Dec;12(6):457–468. doi: 10.1111/j.1751-1097.1970.tb06078.x. [DOI] [PubMed] [Google Scholar]
- Yoakum G., Eisenstark A. Toxicity of L-Tryptophan photoproduct on recombinationless (rec) mutants of Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):653–655. doi: 10.1128/jb.112.1.653-655.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum G., Ferron W., Eisenstark A., Webb R. B. Inhibition of replication gap closure in Escherichia coli by near-ultraviolet light photoproducts of L-tryptophan. J Bacteriol. 1974 Jul;119(1):62–69. doi: 10.1128/jb.119.1.62-69.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



