Abstract
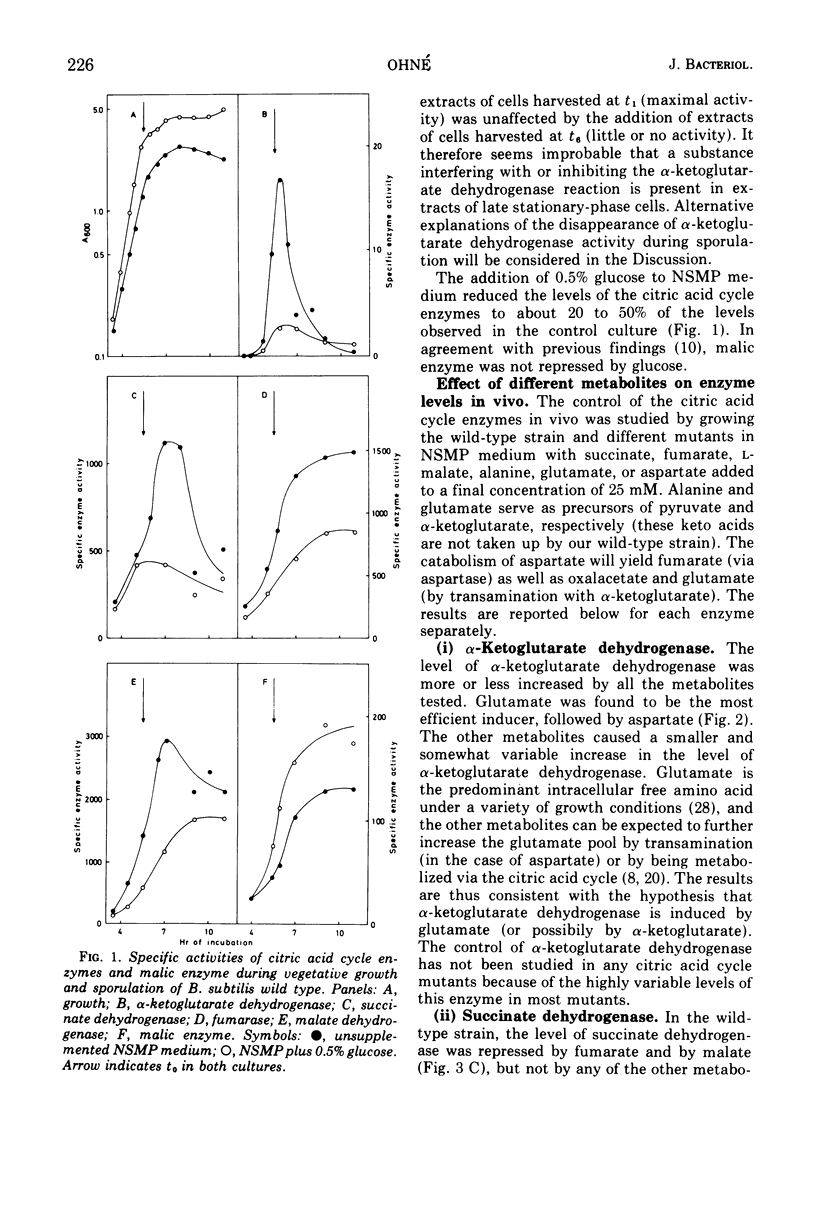
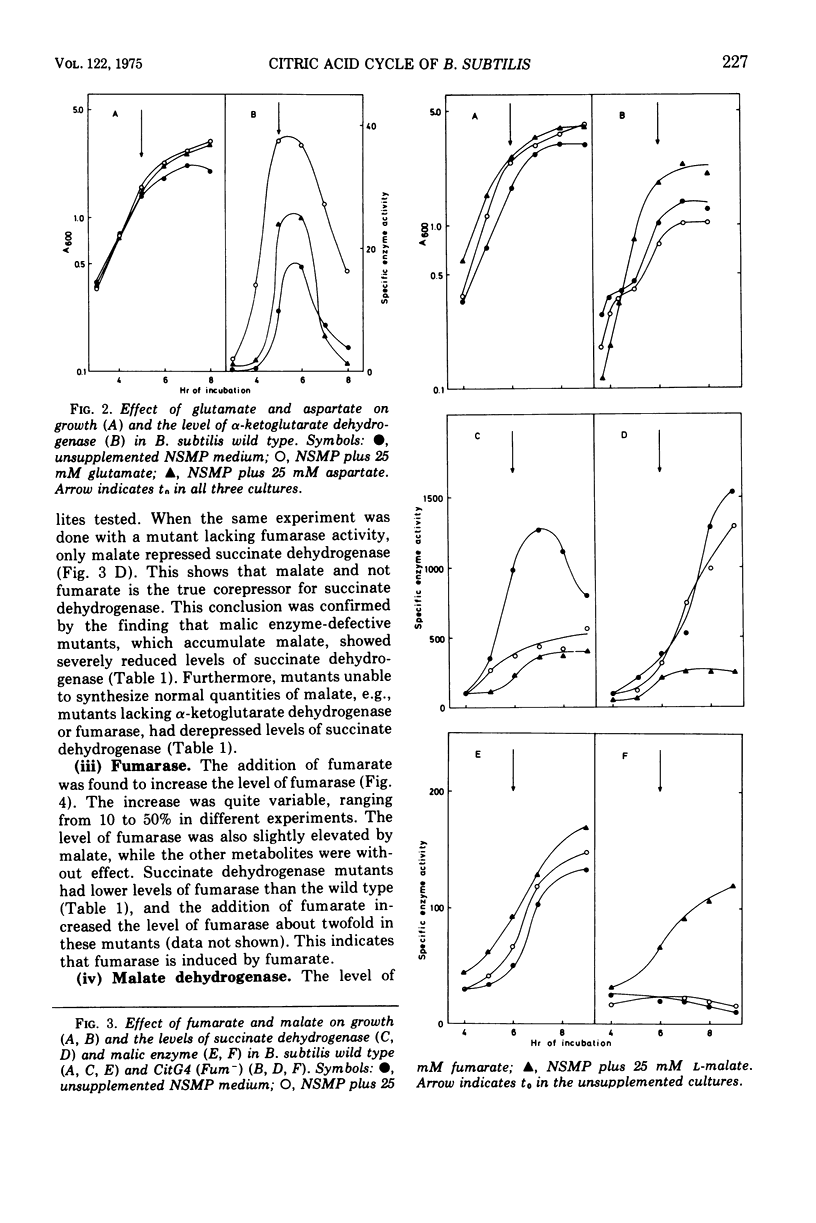
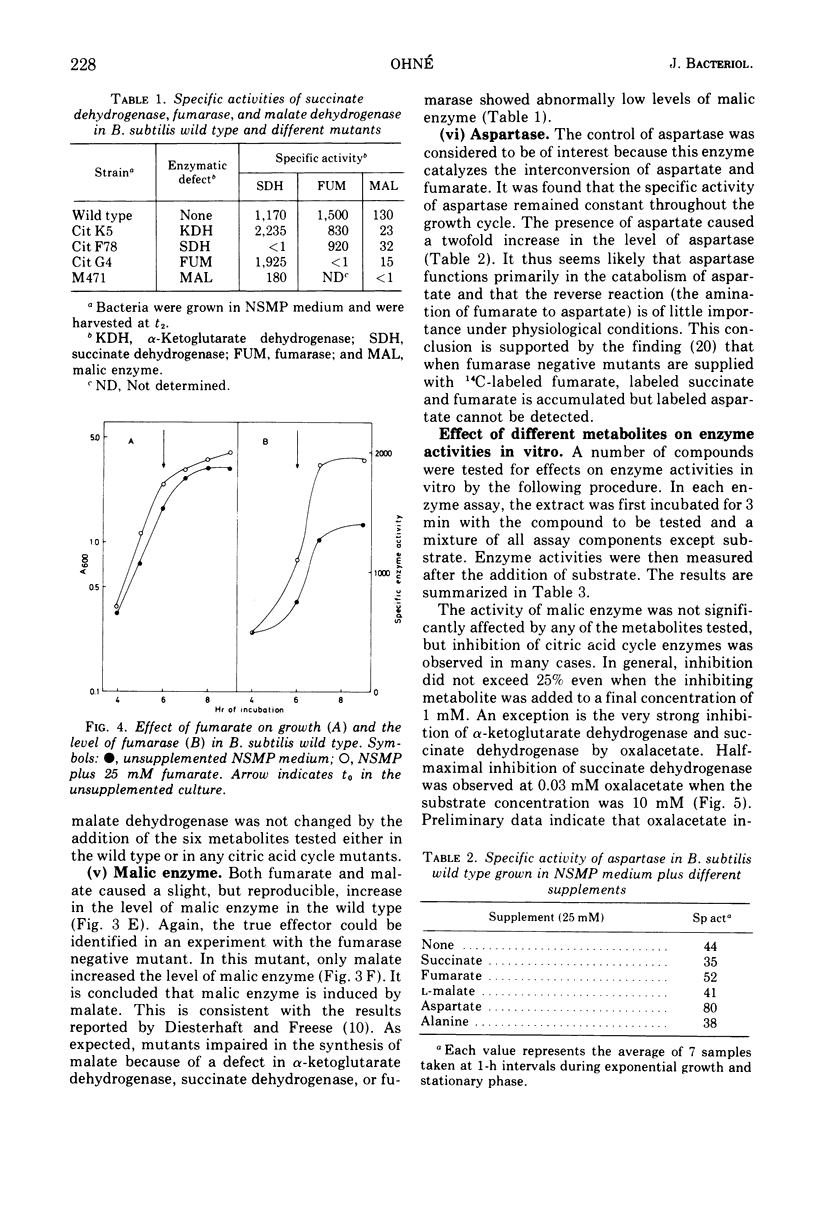
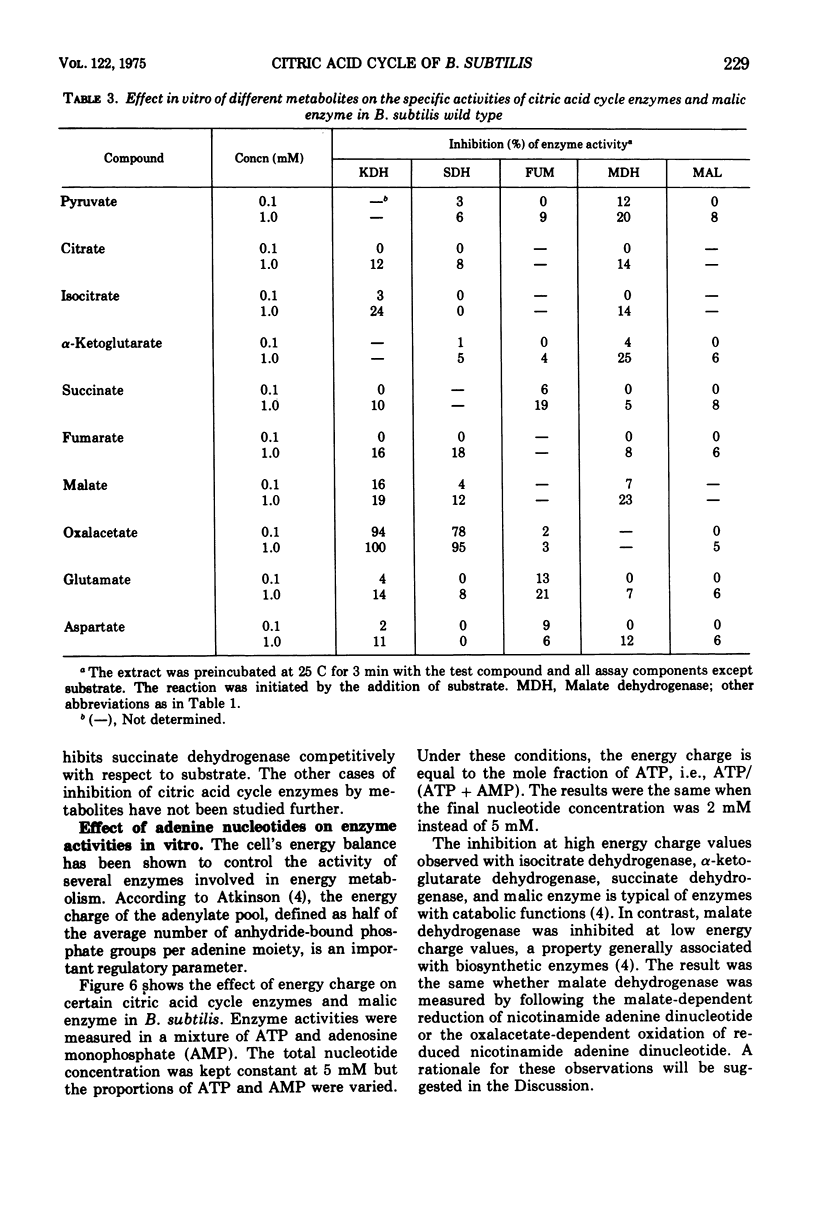
The regulation of alpha-ketogluterate dehydrogenase, succinate dehydrogenase, fumarase, malate dehydrogenase, and malic enzyme has been studied in Bacillus subitilis. The levels of these enzymes increase rapidly during late exponential phase in a complex medium and are maximal 1 to 2 h after the onset of sporulation. Regulation of enzyme synthesis has been studied in the wild type and different citric acid cycle mutants by adding various metabolites to the growth medium. Alpha-ketoglutarate dehydrogenase is induced by glutamate or alpha-ketoglutarate; succinate dehydrogenase is repressed by malate; and fumarase and malic enzyme are induced by fumarate and malate, respectively. The addition of glucose leads to repression of the citric acid cycle enzymes whereas the level of malic enzyme is unaffected. Studies on the control of enzyme activities in vitro have shown that alpha-ketoglutarate dehydrogenase and succinate dehydrogenase are inhibited by oxalacetate. Enzyme activities are also influenced by the energy level, expressed as the energy charge of the adenylate pool. Isocitrate dehydrogenase, alpha-ketoglutarate dehydrogenase, succinate dehydrogenase, and malic enzyme are inhibited at high energy charge values, whereas malate dehydrogenase is inhibited at low energy charge. A survey of the regulation of the citric acid cycle in B.subtilis, based on the present work and previously reported results, is presented and discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
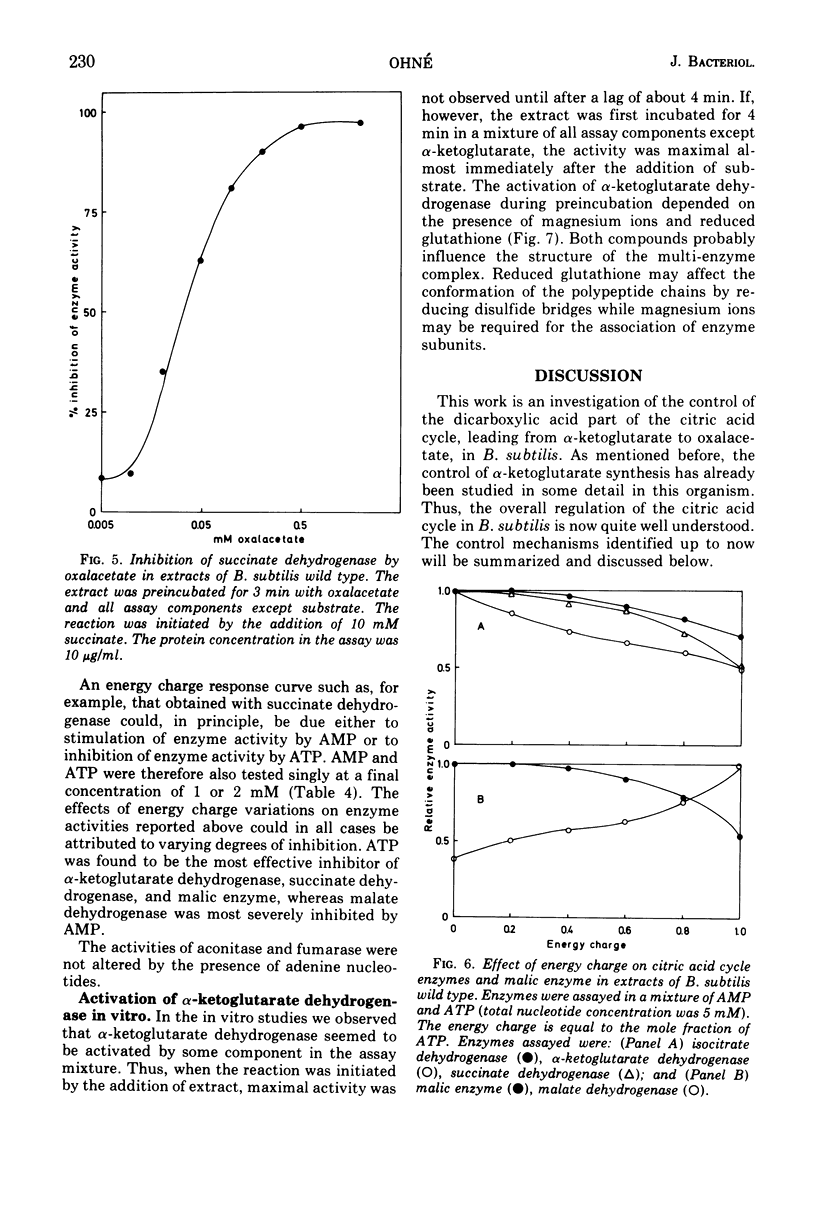
- Ackrell B. A., Kearney E. B., Mayr M. Role 3f oxalacetate in the regulation of mammalian succinate dehydrogenase. J Biol Chem. 1974 Apr 10;249(7):2021–2027. [PubMed] [Google Scholar]
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Bachmann E., Allmann D. W., Green D. E. The membrane systems of the mitochondrion. I. The S fraction of the outer membrane of beef heart mitochondria. Arch Biochem Biophys. 1966 Jul;115(1):153–164. doi: 10.1016/s0003-9861(66)81051-2. [DOI] [PubMed] [Google Scholar]
- Calhoun D. H., Pierson D. L., Jensen R. A. The regulation of tryptophan biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1973 Mar 1;121(2):117–132. doi: 10.1007/BF00277526. [DOI] [PubMed] [Google Scholar]
- Carls R. A., Hanson R. S. Isolation and characterization of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1971 Jun;106(3):848–855. doi: 10.1128/jb.106.3.848-855.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesterhaft M. D., Freese E. Role of pyruvate carboxylase, phosphoenolpyruvate carboxykinase, and malic enzyme during growth and sporulation of Bacillus subtilis. J Biol Chem. 1973 Sep 10;248(17):6062–6070. [PubMed] [Google Scholar]
- FREESE E., PARK S. W., CASHEL M. THE DEVELOPMENTAL SIGNIFICANCE OF ALANINE DEHYDROGENASE IN BACILLUS SUBTILIS. Proc Natl Acad Sci U S A. 1964 Jun;51:1164–1172. doi: 10.1073/pnas.51.6.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Physiological effects of menaquinone deficiency in Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1035–1044. doi: 10.1128/jb.115.3.1035-1044.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechtner V. R., Hanson R. S. Coarse and fine control of citrate synthase from Bacillus subtilis. Biochim Biophys Acta. 1969 Jul 30;184(2):252–262. doi: 10.1016/0304-4165(69)90027-0. [DOI] [PubMed] [Google Scholar]
- Flechtner V. R., Hanson R. S. Regulation of the tricarboxylic acid cycle in bacteria. A comparison of citrate synthases from different bacteria. Biochim Biophys Acta. 1970 Nov 24;222(2):253–264. doi: 10.1016/0304-4165(70)90114-5. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P. The regulation of aconitase and isocitrate dehydrogenase in sporulation mutants of Bacillus subtilis. Biochim Biophys Acta. 1970 Nov 24;222(2):290–298. doi: 10.1016/0304-4165(70)90116-9. [DOI] [PubMed] [Google Scholar]
- Freese E. B., Marks C. L. Developmental block in citric acid cycle mutants of Bacillus subtilis. J Bacteriol. 1973 Dec;116(3):1466–1468. doi: 10.1128/jb.116.3.1466-1468.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Fortnagel U. Growth and sporulation of Bacillus subtilis mutants blocked in the pyruvate dehydrogenase complex. J Bacteriol. 1969 Sep;99(3):745–756. doi: 10.1128/jb.99.3.745-756.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghei O. K., Kay W. W. Properties of an inducible C 4 -dicarboxylic acid transport system in Bacillus subtilis. J Bacteriol. 1973 Apr;114(1):65–79. doi: 10.1128/jb.114.1.65-79.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Mossman M. R. Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):33–41. doi: 10.1016/0304-4165(66)90149-8. [DOI] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. 18. Free amino acids in spores. J Biol Chem. 1970 Mar 10;245(5):1128–1136. [PubMed] [Google Scholar]
- Ohné M. Regulation of aconitase synthesis in Bacillus subtilis: induction, feedback repression, and catabolite repression. J Bacteriol. 1974 Mar;117(3):1295–1305. doi: 10.1128/jb.117.3.1295-1305.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohné M., Rutberg B., Hoch J. A. Genetic and biochemical characterization of mutants of Bacillus subtilis defective in succinate dehydrogenase. J Bacteriol. 1973 Sep;115(3):738–745. doi: 10.1128/jb.115.3.738-745.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Regulation of beta-galactosidase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1968 Oct 25;243(20):5420–5427. [PubMed] [Google Scholar]
- Rutberg B., Hoch J. A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochikubo K. Changes in terminal respiratory pathways of Bacillus subtilis during germination, outgrowth and vegetative growth. J Bacteriol. 1971 Nov;108(2):652–661. doi: 10.1128/jb.108.2.652-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA A. ENZYMIC PROPERTIES OF MALATE DEHYDROGENASE OF BACILLUS SUBTILIS. J Biol Chem. 1965 Mar;240:1118–1124. [PubMed] [Google Scholar]
- YOSHIDA A. PURIFICATION AND CHEMICAL CHARACTERIZATION OF MALATE DEHYDROGENASE OF BACILLUS SUBTILIS. J Biol Chem. 1965 Mar;240:1113–1117. [PubMed] [Google Scholar]


