Abstract
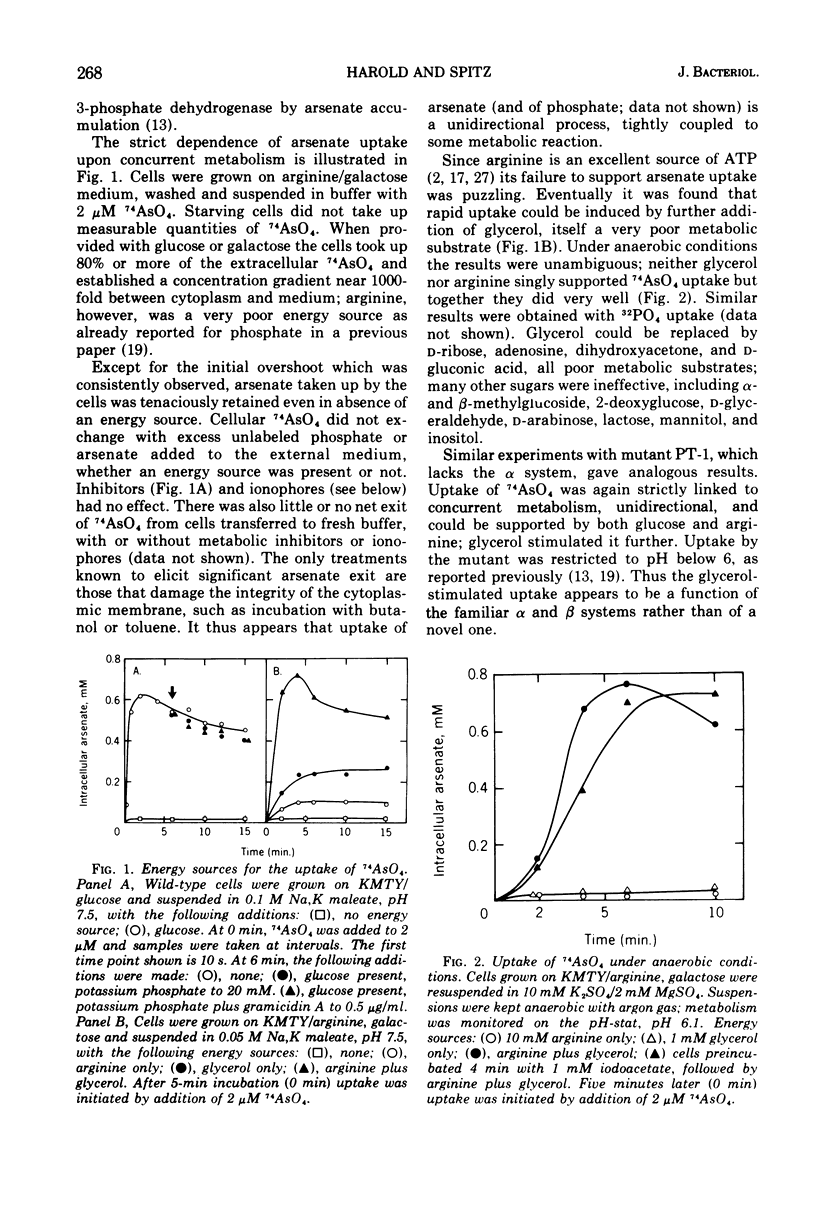
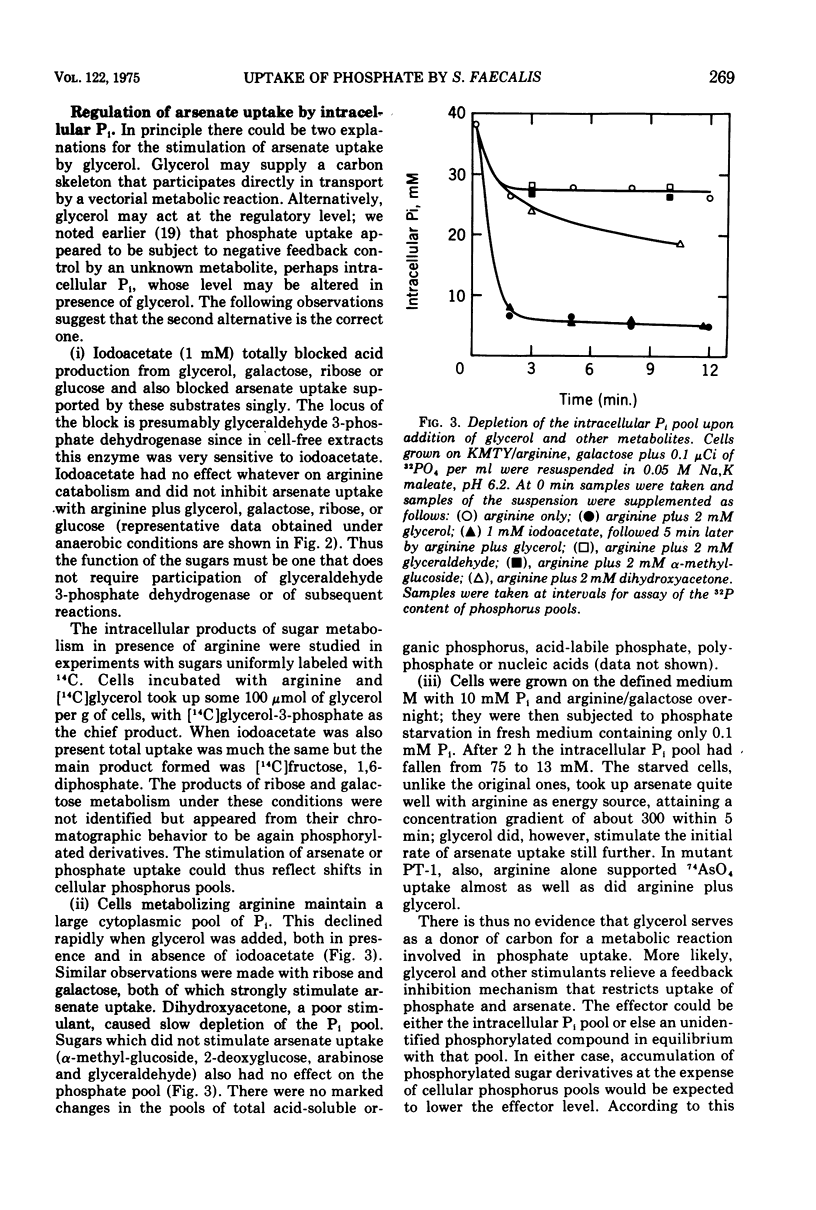
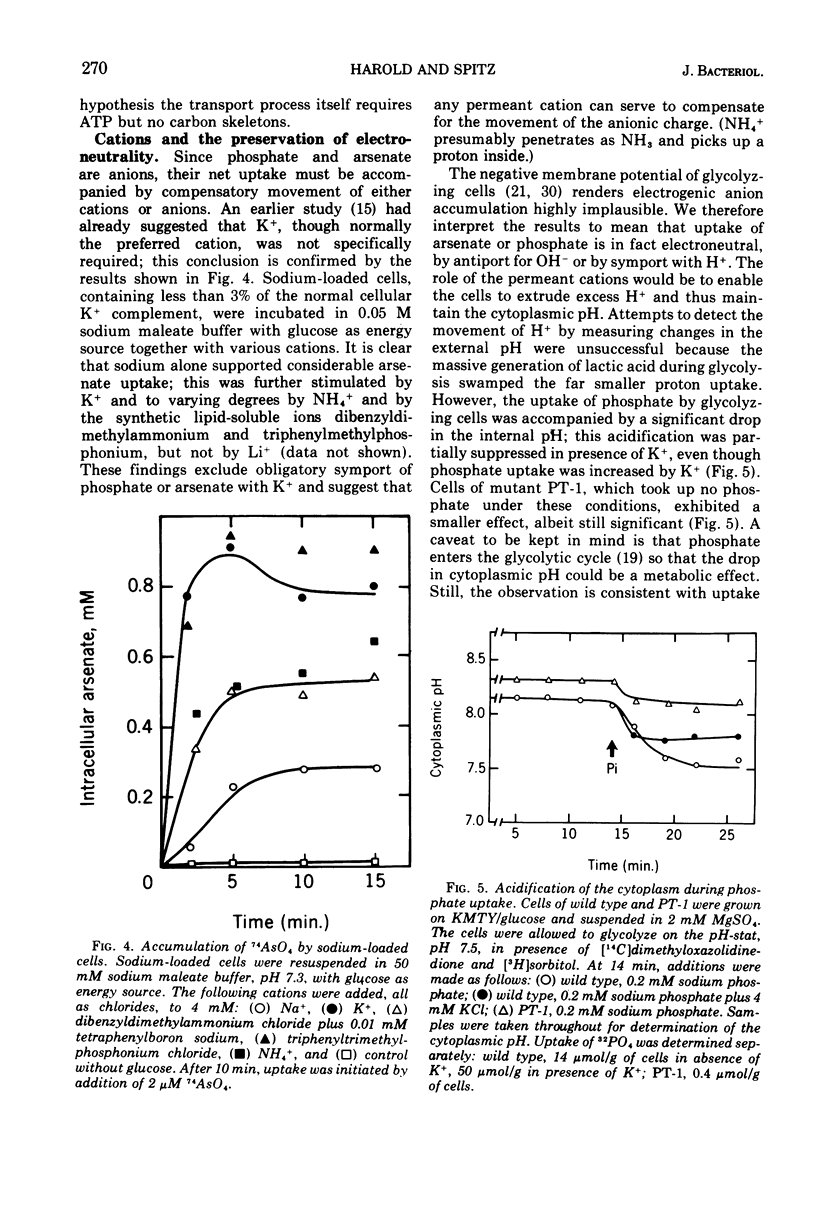
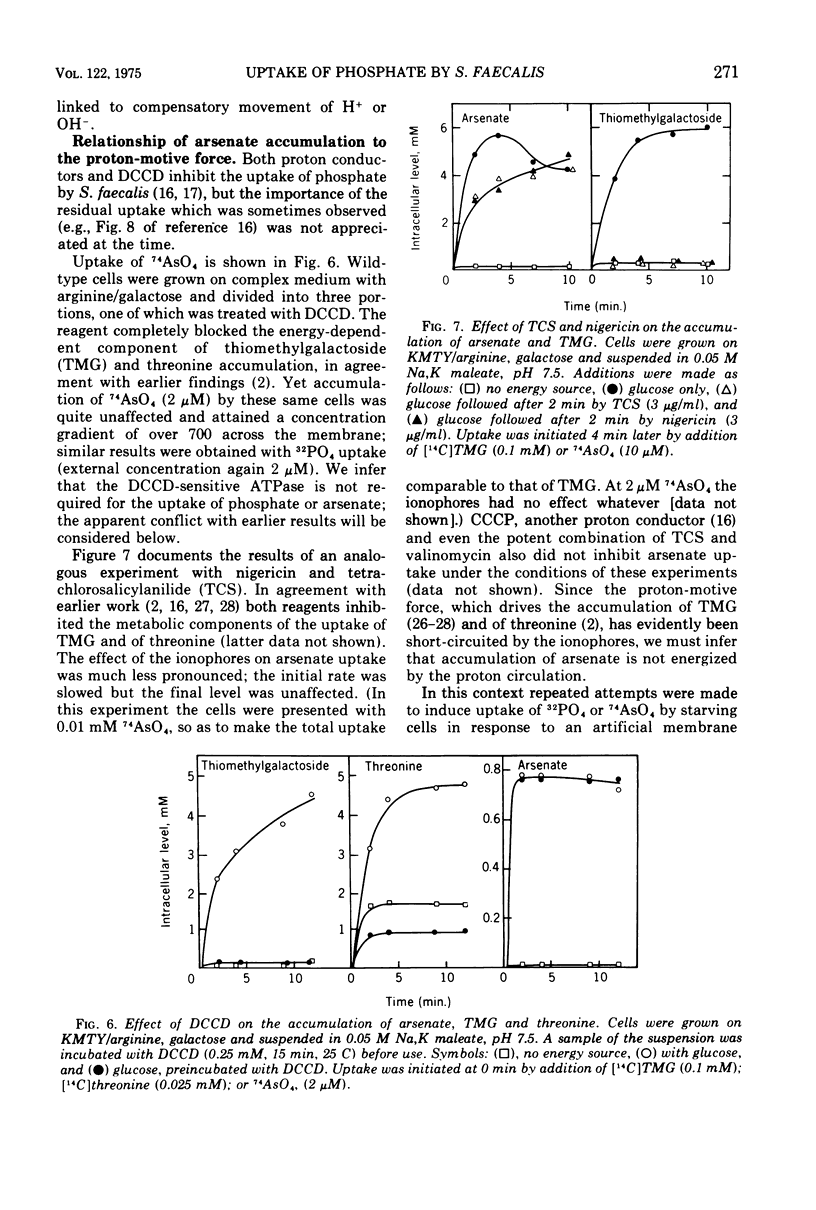
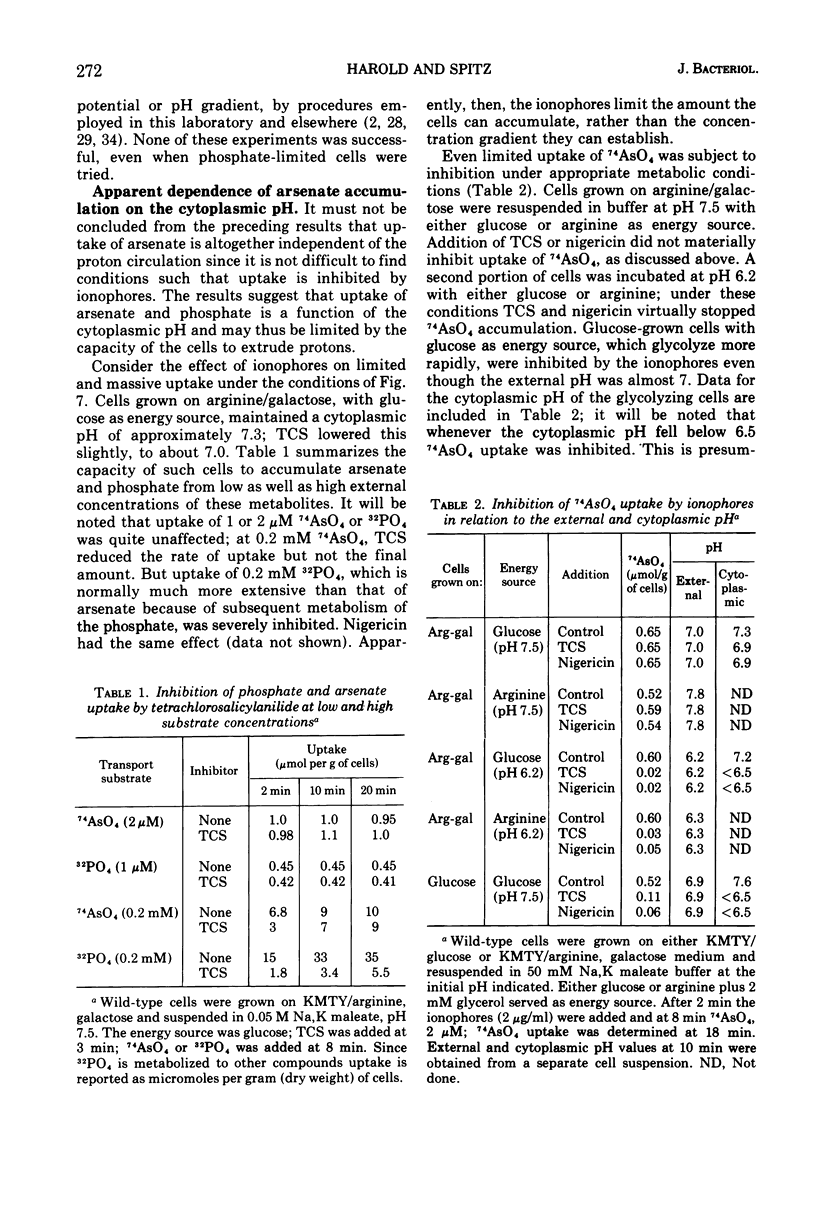
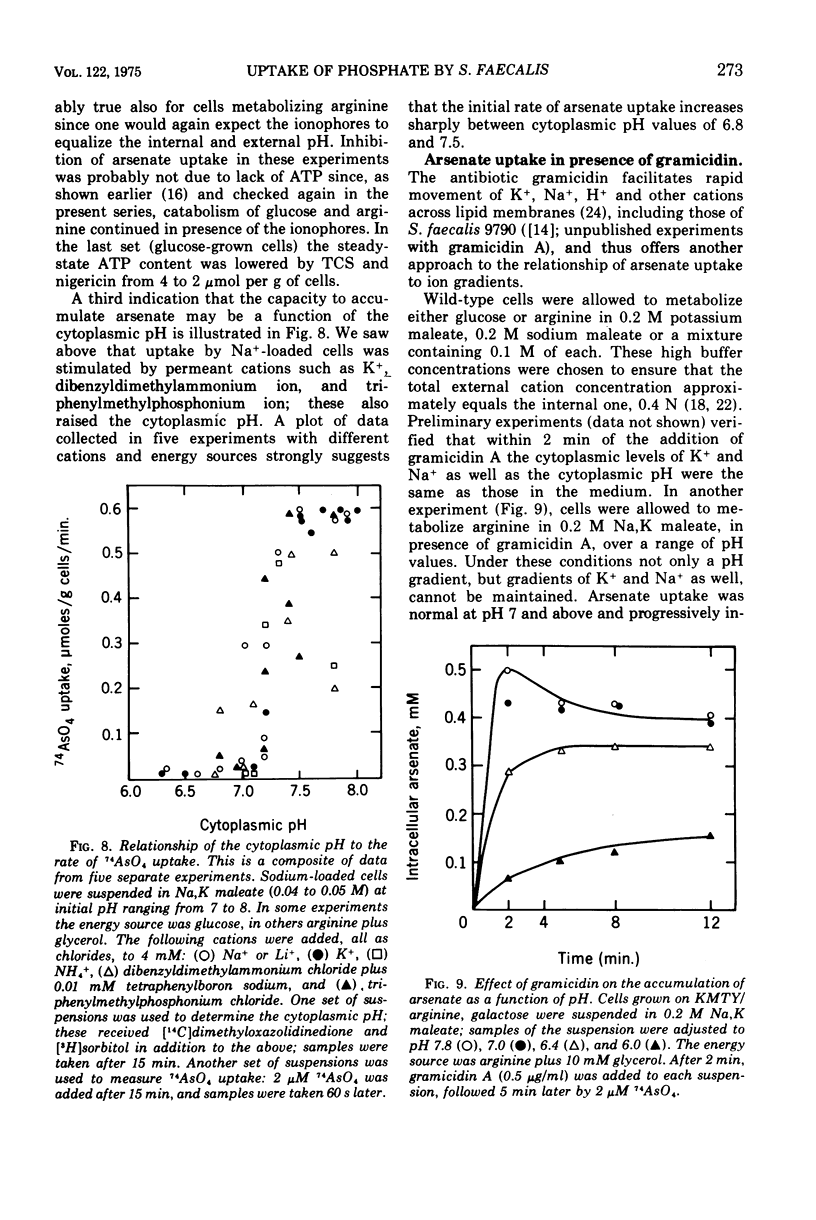
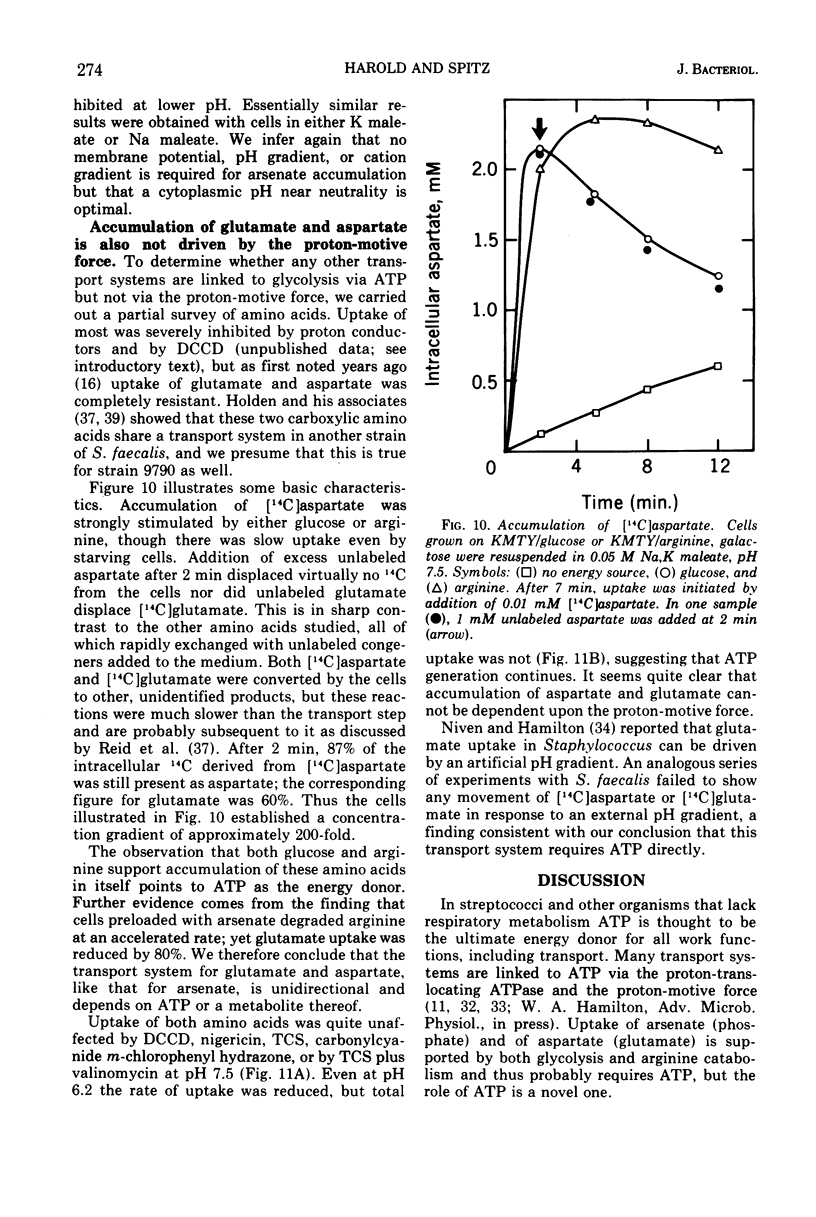
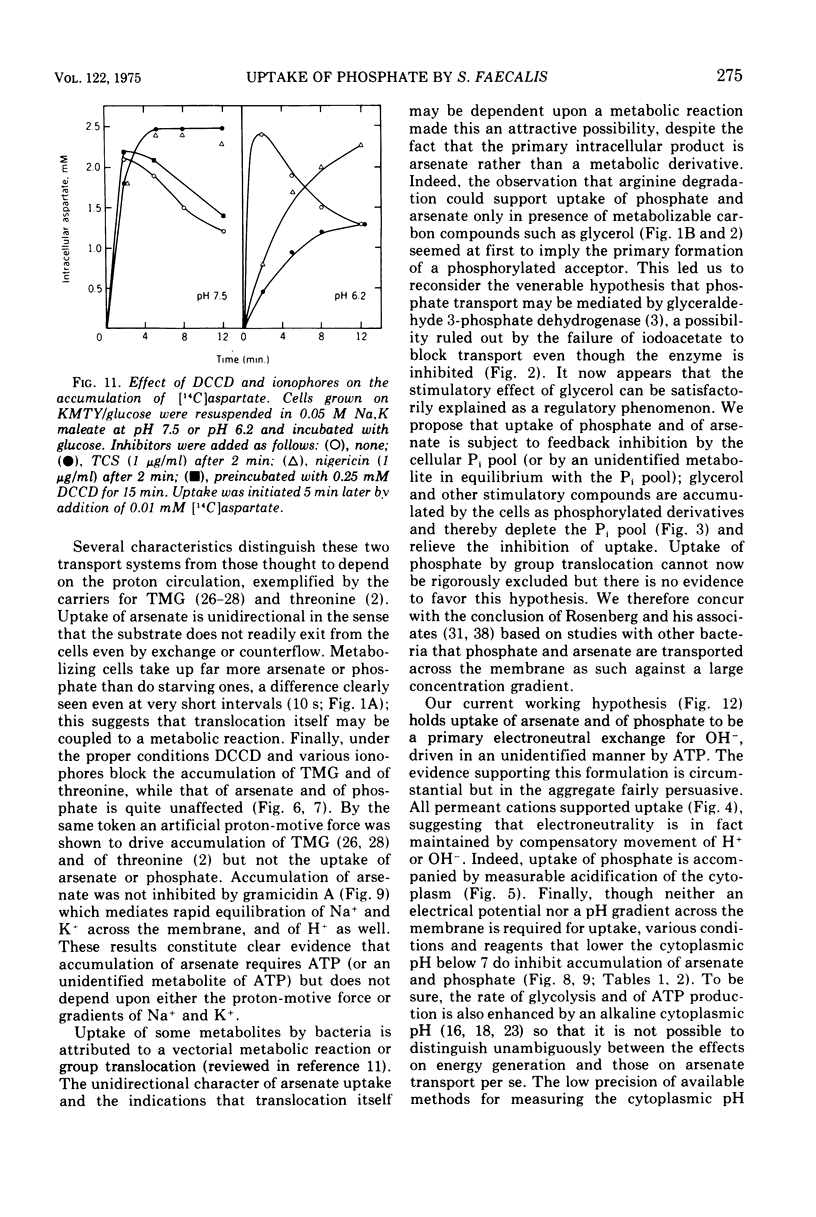
Uptake of arsenate and phosphate by Streptococcus faecalis 9790 is strictly dependent on concurrent energy metabolism and essentially unidirectional. targinine supports uptake only in presence of glycerol or related substances; glycerol is not directly involved in transport but depletes the cellular orthophosphate pool and thus relieves feedback inhibition of transport. Uptake of phosphate and arsenate is stimulated by K+ and by other permeant cations. The results suggest that electroneutrality is preserved by compensatory movement of either H+ or OH minus. Ionophores and N,N'-dicyclohexylcarbodiimide, which prevent establishment of a proton motive force, block the accumulation of thiomethylgalactoside and of threonine but not that of arsenate or phosphate. We conclude that arsenate accumulation requires adenosine 5'-triphosphate but is not driven by the proton-motive force. However, conditions and reagents that lower the cytoplasmic pH do inhibit accumulation of arsenate and phosphate, suggesting that uptake depends on the capacity of the cells to maintain a neutral or alkaline cytoplasm. We therefore propose that phosphate accumulation is an electroneutral exchange for OH driven by adenosine 5'-triphosphate or by a metabolite thereof. Accumulation of aspartate and glutamate also requires adenosine 5'-triphosphate but not the proton-motive force and may involve a similar mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A., Smith J. B., Baron C. Carbodiimide-resistant membrane adenosine triphosphatase in mutants of Streptococcus faecalis. I. Studies of the mechanism of resistance. J Biol Chem. 1972 Mar 10;247(5):1484–1488. [PubMed] [Google Scholar]
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- BARTLETT G. R. Organization of red cell glycolytic enzymes; cell coat phosphorus transfer. Ann N Y Acad Sci. 1958 Oct 13;75(1):110–114. doi: 10.1111/j.1749-6632.1958.tb36855.x. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Borst-Pauwels G. W., Dobbelmann J. The mechanism of inhibition of anaerobic phosphate uptake by fatty acids in yeast. Biochim Biophys Acta. 1972 Dec 1;290(1):348–354. doi: 10.1016/0005-2736(72)90077-6. [DOI] [PubMed] [Google Scholar]
- Cowell J. L. Energetics of glycylglycine transport in Escherichia coli. J Bacteriol. 1974 Oct;120(1):139–146. doi: 10.1128/jb.120.1.139-146.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J. Mechanism of energy coupling for transport of D-ribose in Escherichia coli. J Bacteriol. 1974 Oct;120(1):295–303. doi: 10.1128/jb.120.1.295-303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAROLD F. M., HAROLD R. L., ABRAMS A. A MUTANT OF STREPTOCOCCUS FAECALIS DEFECTIVE IN PHOSPHATE UPTAKE. J Biol Chem. 1965 Jul;240:3145–3153. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Effects of nigericin and monactin on cation permeability of Streptococcus faecalis and metabolic capacities of potassium-depleted cells. J Bacteriol. 1968 Mar;95(3):816–823. doi: 10.1128/jb.95.3.816-823.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Gramicidin, valinomycin, and cation permeability of Streptococcus faecalis. J Bacteriol. 1967 Jul;94(1):53–60. doi: 10.1128/jb.94.1.53-60.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Interaction of arsenate with phosphate-transport systems in wild- type and mutant Streptococcus faecalis. J Bacteriol. 1966 Jun;91(6):2257–2262. doi: 10.1128/jb.91.6.2257-2262.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Pavlasova E. Extrusion of sodium and hydrogen ions as the primary process in potassium ion accumulation by Streptococcus faecalis. J Bacteriol. 1970 Jan;101(1):152–159. doi: 10.1128/jb.101.1.152-159.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol Rev. 1966 Dec;30(4):772–794. doi: 10.1128/br.30.4.772-794.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Levin E. Lactic acid translocation: terminal step in glycolysis by Streptococcus faecalis. J Bacteriol. 1974 Mar;117(3):1141–1148. doi: 10.1128/jb.117.3.1141-1148.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. I. The membrane potential. J Membr Biol. 1972;8(1):27–44. doi: 10.1007/BF01868093. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. II. Proton and sodium extrusion. J Membr Biol. 1972;8(1):45–62. doi: 10.1007/BF01868094. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Pavlasová E., Baarda J. R. A transmembrane pH gradient in Streptococcus faecalis: origin, and dissipation by proton conductors and N,N'-dicyclohexylcarbodimide. Biochim Biophys Acta. 1970;196(2):235–244. doi: 10.1016/0005-2736(70)90011-8. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hladky S. B. Ion transport across thin lipid membranes: a critical discussion of mechanisms in selected systems. Q Rev Biophys. 1972 May;5(2):187–282. doi: 10.1017/s0033583500000883. [DOI] [PubMed] [Google Scholar]
- Huygen P. L., Borst-Pauwels G. W. The effect of N,N'-dicyclohexylcarbodiimide on anaerobic and aerobic phosphate uptake by baker's yeast. Biochim Biophys Acta. 1972 Nov 17;283(2):234–238. doi: 10.1016/0005-2728(72)90239-3. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Galactoside accumulation associated with ion movements in Streptococcus lactis. Biochem Biophys Res Commun. 1972 Nov 1;49(3):615–620. doi: 10.1016/0006-291x(72)90455-x. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Role of metabolic energy in the transport of -galactosides by Streptococcus lactis. J Bacteriol. 1972 Feb;109(2):784–789. doi: 10.1128/jb.109.2.784-789.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Klingenberg M., Palmieri F., Quagliariello E. Quantitative correlation between the distribution of anions and the pH difference across the mitochondrial membrane. Eur J Biochem. 1970 Dec;17(2):230–238. doi: 10.1111/j.1432-1033.1970.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Laris P. C., Pershadsingh H. A. Estimations of membrane potentials in Streptococcus faecalis by means of a fluorescent probe. Biochem Biophys Res Commun. 1974 Apr 8;57(3):620–626. doi: 10.1016/0006-291x(74)90591-9. [DOI] [PubMed] [Google Scholar]
- Medveczky N., Rosenberg H. Phosphate transport in Escherichia coli. Biochim Biophys Acta. 1971 Aug 13;241(2):494–506. doi: 10.1016/0005-2736(71)90048-4. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Performance and conservation of osmotic work by proton-coupled solute porter systems. J Bioenerg. 1973 Jan;4(1):63–91. doi: 10.1007/BF01516051. [DOI] [PubMed] [Google Scholar]
- Niven D. F., Hamilton W. A. Mechanisms of energy coupling to the transport of amino acids by Staphylococcus aureus. Eur J Biochem. 1974 May 15;44(2):517–522. doi: 10.1111/j.1432-1033.1974.tb03510.x. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Cools A., van Dam K. The transport of Krebs-cycle intermediates in Azotobacter vinelandii under various metabolic conditions. Biochim Biophys Acta. 1973 Aug 9;318(1):91–104. doi: 10.1016/0005-2736(73)90339-8. [DOI] [PubMed] [Google Scholar]
- Reid K. G., Utech N. M., Holden J. T. Multiple transport components for dicarboxylic amino acids in Streptococcus faecalis. J Biol Chem. 1970 Oct 25;245(20):5261–5272. [PubMed] [Google Scholar]
- Rosenberg H., Medveczky N., La Nauze J. M. Phosphate transport in Bacillus cereus. Biochim Biophys Acta. 1969 Oct 14;193(1):159–167. doi: 10.1016/0005-2736(69)90069-8. [DOI] [PubMed] [Google Scholar]
- Utech N. M., Reid K. G., Holden J. T. Properties of a dicarboxylic amino acid transport-deficient mutant of Streptococcus faecalis. J Biol Chem. 1970 Oct 25;245(20):5273–5280. [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. The catalytic effect of molybdate on the hydrolysis of organic phosphate bonds. Biochem J. 1951 Aug;49(3):286–292. [PMC free article] [PubMed] [Google Scholar]



