Abstract
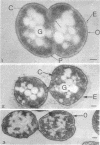
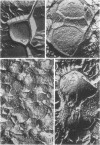
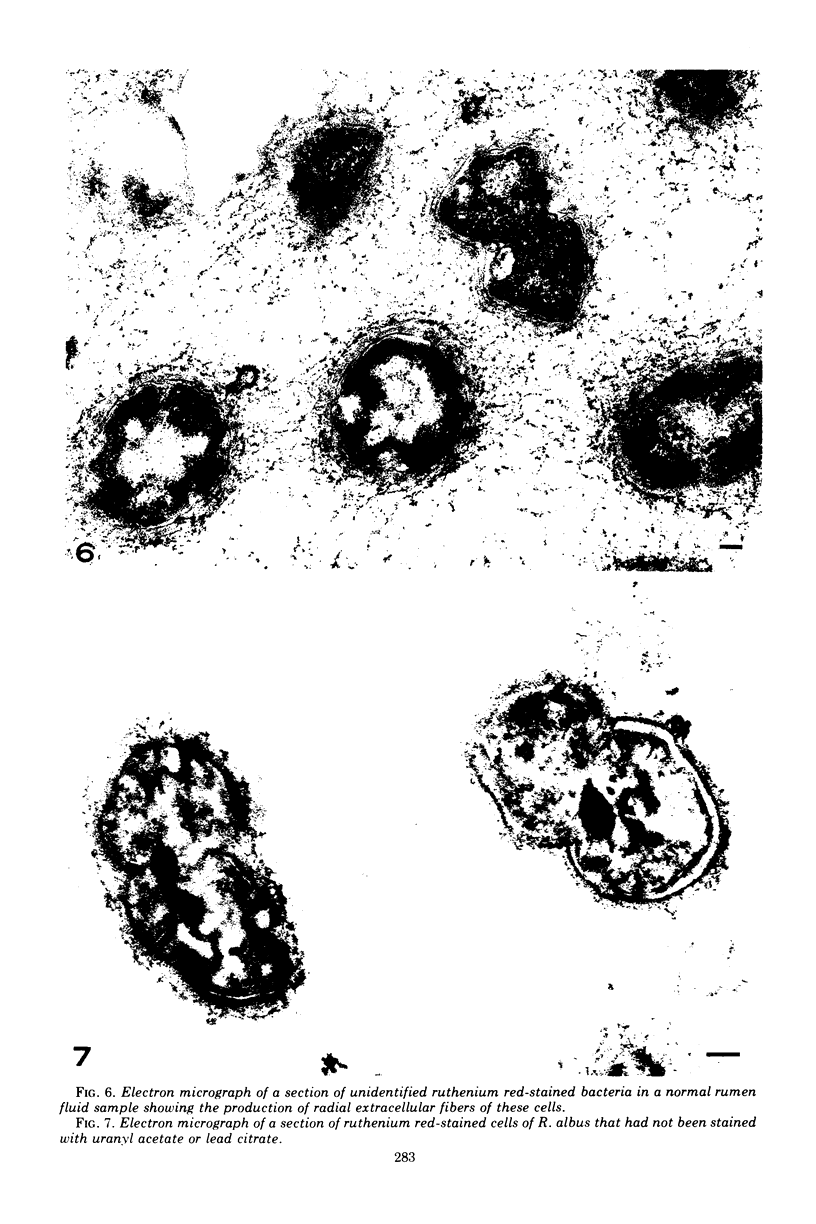
Morphological studies have shown that cells of the anaerobic rumen bacterium Ruminococcus albus have electron-translucent granules of reserve carbohydrate in their cytoplasm, and that they have a polysaccharide "coat" layer external to their gram-negative cell wall. This coat layer, which stains specifically with ruthenium red, forms a compact mat of fibers adjacent to the cell, and fibrous elements also project as much as 0.6 mum from the cells. These radial fibers are clearly visualized by freeze-etching, and can be seen to extend throughout the extensive intercullular space in centrifuged pellets of these bacteria. Cells of R. albus adhere to cellulose fibers added to the culture medium, and the coat material is seen to mediate this adhesion in addition to its function in the general protection of these cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H. C., Cota-Robles E. H., Casida L. E. Microflora of soil as viewed by transmission electron microscopy. Appl Microbiol. 1972 Mar;23(3):637–648. doi: 10.1128/am.23.3.637-648.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill D. L., Casida L. E., Jr Microflora of soil as viewed by freeze-etching. J Bacteriol. 1973 Jun;114(3):1319–1327. doi: 10.1128/jb.114.3.1319-1327.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Localization of alkaline phosphatase in three gram-negative rumen bacteria. J Bacteriol. 1973 Oct;116(1):424–440. doi: 10.1128/jb.116.1.424-440.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Hironaka R., Roberts D. W., Costerton J. W. Cytoplasmic glycogen inclusions in cells of anaerobic gram-negative rumen bacteria. Can J Microbiol. 1973 Dec;19(12):1501–1506. doi: 10.1139/m73-244. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Damgaard H. N., Cheng K. J. Cell envelope morphology of rumen bacteria. J Bacteriol. 1974 Jun;118(3):1132–1143. doi: 10.1128/jb.118.3.1132-1143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Costerton J. W., MacLeod R. A. Demonstration by freeze-etching of a single cleavage plane in the cell wall of a gram-negative bacterium. J Bacteriol. 1971 May;106(2):659–671. doi: 10.1128/jb.106.2.659-671.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. POLYSACCHARIDE STORAGE AND GROWTH EFFICIENCY IN RUMINOCOCCUS ALBUS. J Bacteriol. 1963 Oct;86:848–854. doi: 10.1128/jb.86.4.848-854.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. C., Roth I. L., Sanders W. M., 3rd Electron microscopic study of a slime layer. J Bacteriol. 1969 Jul;99(1):316–325. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laishley E. J., MacAlister T. J., Clements I., Young C. Isolation and morphology of native intracellular polyglucose granules from Clostridium pasteurianum. Can J Microbiol. 1973 Aug;19(8):991–994. doi: 10.1139/m73-157. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Moor H. Freeze-etching. Int Rev Cytol. 1969;25:391–412. doi: 10.1016/s0074-7696(08)60209-0. [DOI] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967 Oct;35(1):37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Pruul H. Protective role of smooth lipopolysaccharide in the serum bactericidal reaction. Infect Immun. 1971 Dec;4(6):764–771. doi: 10.1128/iai.4.6.764-771.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W. Localization of somatic antigen on gram-negative bacteria using ferritin antibody conjugates. Ann N Y Acad Sci. 1966 Jun 30;133(2):292–298. doi: 10.1111/j.1749-6632.1966.tb52372.x. [DOI] [PubMed] [Google Scholar]
- Shilo M. Lysis of blue-green algae by myxobacter. J Bacteriol. 1970 Oct;104(1):453–461. doi: 10.1128/jb.104.1.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. R., Yu I., Hungate R. E. Factors affecting cellulolysis by Ruminococcus albus. J Bacteriol. 1973 May;114(2):729–737. doi: 10.1128/jb.114.2.729-737.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. The ultrastructure of the capsules of Diplococcus pneumoniae and Klebsiella pneumoniae stained with ruthenium red. J Gen Microbiol. 1973 Jan;74(1):21–31. doi: 10.1099/00221287-74-1-21. [DOI] [PubMed] [Google Scholar]
- Watson S. W., Remsen C. C. Macromolecular subunits in the walls of marine nitrifying bacteria. Science. 1969 Feb 14;163(3868):685–686. doi: 10.1126/science.163.3868.685. [DOI] [PubMed] [Google Scholar]