Abstract
Single-channel recordings were obtained from Chinese hamster ovary cells transfected with the N-methyl-d-aspartate (NMDA) receptor subunit NR1 in combination with NR2A, NR2B, NR2C, or NR2A/NR2B. NMDA-activated currents were recorded under control conditions and in the presence of a thiol reductant (DTT), an oxidant (5,5′-dithio-bis[2-nitrobenzoic acid], DTNB), or the noncompetitive antagonist CP101,606 (CP). For all subunit combinations, DTT increased the frequency of channel opening when compared with DTNB. In addition, channels obtained from NR1/NR2A-transfected cells also exhibited a pronounced difference in mean open dwell-time between redox conditions. CP dramatically reduced both the open dwell-time and frequency of channel opening of NR1/NR2B-containing receptors, but only modestly inhibited NR1/NR2A and NR1/NR2C channel activity. A small number of patches obtained from cells transfected with NR1/NR2A/NR2B had channels with properties intermediate to NR1/NR2A and NR1/NR2B receptors, including insensitivity to CP block but redox properties similar to NR1/NR2B, consistent with the coassembly of NR2A with NR2B. Hence, NMDA receptors containing multiple types of NR2 subunits can have functionally distinguishable attributes.
The N-methyl-d-aspartate (NMDA) receptor is a ligand-gated ion channel involved in excitatory neurotransmission, synaptic plasticity, and neuronal cell death. Two families of NMDA receptor subunits exist: the NR1 subunit (1), which is found in eight alternatively spliced isoforms (2), and the NR2 subunit, for which four subtypes have been described (NR2A-D), each one being a different gene product (3–7). Functional NR1 homomers apparently can be assembled in frog oocytes (1), whereas receptors in mammalian cells likely are formed by the coassembly of the NR1 subunit with at least one type of NR2 subunit (8–13). Although it recently has been suggested that functional NMDA channels most likely contain two NR1 subunits (ref. 14, but see ref. 15), the number of NR2 subunits that coassemble with NR1 has yet to be determined.
Individual NR2 subunits coassembling with NR1 produce recombinant NMDA receptors with varying sensitivity to pharmacological agents (16–19) and different channel permeation and kinetic properties (4, 6, 20–24). Hence, the number and type of NR2 subunits in a single receptor likely will influence its overall function. In the present study, we have developed a series of profiles, based on unique biophysical and pharmacological properties, for four different combinations of NR1, NR2A, NR2B, and NR2C subunits transiently expressed in Chinese hamster ovary (CHO) cells (25).
MATERIALS AND METHODS
Transfection Protocol.
CHO-K1 cells were grown in Ham’s F-12 nutrient medium with 10% fetal bovine serum and 1 mM glutamine and passaged at a 1:10 dilution when 80% confluent, approximately every 2 days. The cDNAs for the NMDA subunits previously were subcloned into expression vectors (25). The vector for a positive transfection marker, green fluorescent protein (GFP), was formed by ligating the 750-bp BssHII–NotI fragment from TU65 (26) into the MluI–NotI sites of pCI (Promega) to generate pCI/GFP. CHO cells were seeded at 3 × 105 cells per well in 6-well plates approximately 24 hr before transfection with 1.3 μg of total DNA and 6 μl of Lipofectamine (GIBCO/BRL) in 1 ml of serum-free CHO media per 35-mm dish. The ratio of pCI/GFP to total DNA was 1:4.3, and the ratio of NR1a to NR2 was always 1:3 (27). When two different NR2 subunits were cotransfected, their ratio was 1:1. After a 4- to 5-hr incubation at 37°C, cells were refed with CHO medium containing 1 mM 5,7-dichlorokynurenic acid to prevent cell death (25). Cells were used for recording 40–50 hr after transfection.
Patch-Clamp Recordings.
Recordings were performed at ≈25°C on outside-out patches with 10–15 MΩ electrodes. Signals were amplified with an Axopatch 200 (Axon Instruments, Foster City, CA), filtered at 2 kHz, stored on videotape, and later digitized at 10 kHz (Digidata 1200, Axon Instruments). The extracellular solution contained 150 mM NaCl, 2.8 mM KCl, 1.0 mM CaCl2, 10 mM Hepes, 0.01 mM glycine, pH adjusted to 7.2 with 0.3 N NaOH. The pipette solution contained 140 mM CsF, 10 mM EGTA, 1.0 mM CaCl2, 10 mM Hepes, pH adjusted to 7.2 with CsOH. NMDA, DTT, DTNB, and the novel ifenprodil analog CP101,606 (CP, mesylate salt; ref. 28) were applied to the patch by complete bath exchange.
Data Analysis.
Single-channel analysis was performed using pClamp6 software (Axon Instruments) using a 50% threshold detection criteria. In total, data were gathered from 48 patches obtained from transfected CHO cells, from at least 75 separate transfection experiments. Data from patches lost before the completion of a treatment protocol (>100) were not used as each patch served as its own control. In addition, a large number of patches from green fluorescent protein-positive CHO cells (>500) contained no NMDA channels. Normally, 200–500 events were analyzed per single treatment, although some records contained as many as 1,800 events. Multiple openings, when present, constituted <6% of all events for a given treatment; open channel probability values are given as nPo, n being the number of channels in the patch. Amplitude histograms were most commonly fit with single Gaussians. Most open dwell-time histograms were best-fit with a single exponential function using a simplex maximum likelihood routine on log-transformed binned data (6 bins/decade). A chi-squared test was used to determine the simplest fit of the data. When an open dwell-time histogram was better-fit by the sum of two exponentials (only 5% of all histograms), the arithmetic mean open time was used for the comparisons of means. Events briefer than 180 μs (twice the rise time of the filter) were ignored. Results are expressed as mean ± SEM.
Our approach to evaluate kinetic differences between the various subunit combinations required a careful determination of open dwell-time. Hence, to validate our analysis method we evaluated the effects of Mg2+ on NMDA channels in outside-out patches excised from cortical neurons. We used various Mg2+ concentrations (0, 10, 50, and 100 μM) at three holding potentials in three separate patches. After the calculation of the blocking and unblocking rate constants, the KDs for Mg2+ block obtained were 34.3 ± 12.3 μM at −80 mV, 75.0 ± 11.6 μM at −60 mV, and 288.3 ± 91.2 μM at −40 mV, values similar to those obtained previously (29).
RESULTS
Functional Analysis of NR1/NR2A, NR1/NR2B, and NR1/NR2C-Containing Channels.
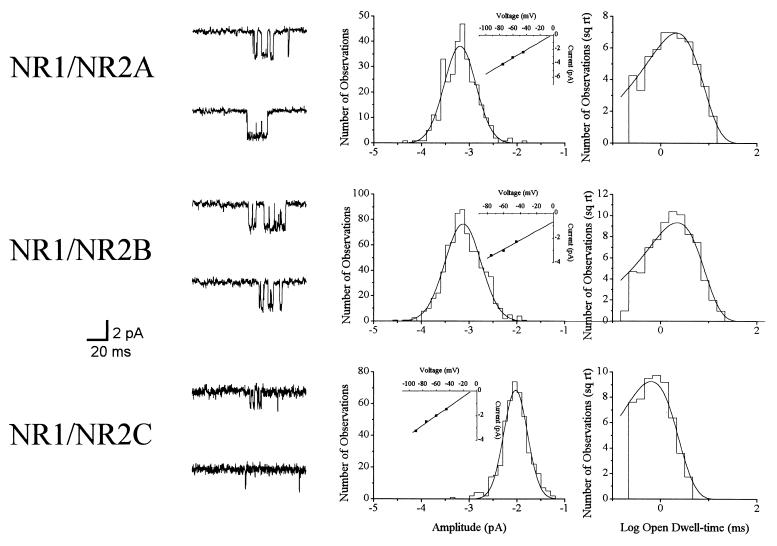
NMDA receptor channel activity was measured in patches excised from CHO cells transfected with either NR1/NR2A (n = 11), NR1/NR2B (n = 13), or NR1/NR2C (n = 3). Events were elicited by 10 μM NMDA at −60 mV in patches not previously exposed to any other treatment (Fig. 1). Amplitude histograms revealed a mean unitary current of −3.4 ± 0.1 pA for NR1/NR2A receptor channels. The mean time constant for the exponential functions fitting the open dwell-time histograms was 2.6 ± 0.3 ms for this subunit combination. NR1/NR2B channels had an amplitude of −3.5 ± 0.1 pA and an open dwell-time constant of 2.9 ± 0.3 ms. With similar measurements at −75 mV and −45 mV, we estimated the slope conductances to be near 50 pS for both the NR1/NR2A and NR1/NR2B receptor configurations. The amplitude of an NR1/NR2C channel was −2.0 ± 0.1 pA, with a conductance of 35 pS, and an open dwell-time constant of 0.7 ± 0.1 ms. All of these values are in good agreement with previous data obtained with other expression systems (21, 30).
Figure 1.
Single-channel properties of NMDA receptors expressed in CHO cells. (Left) Representative NR1/NR2A, NR1/NR2B, and NR1/NR2C channels recorded from outside-out patches during continuous exposure to 10 μM NMDA at −60 mV. (Center) The amplitude histograms obtained from these events were fit with single Gaussian functions. Similar measurements recorded at other holding voltages were used to generate I–V relations (Insets) with slope conductances of 55 pS for NR1/NR2A, 47 pS for NR1/NR2B, and 36 pS for NR1/NR2C. (Right) The open dwell-time distributions were best-fit with single exponential functions with time constants of 2.2, 2.0, and 0.8 ms for the three subunit combinations, respectively. Similar events were recorded in different patches for each receptor configuration in the absence of any other drug treatments.
Redox Modulation of Recombinant NMDA Receptors.
The NMDA receptor is modulated by sulfhydryl redox reagents such that reductants can potentiate NMDA-induced responses, whereas oxidants can depress native responses and reverse the effects of reducing agents (31, 32). Molecular studies have established the presence of two redox sites on the NMDA receptor, one in the putative extracellular loop between M2 and M3 of NR1 (33) and one in the amino terminus of NR2A (34). We sought to determine whether the single-channel behavior after redox treatments would differ between NR1/NR2A receptors, which theoretically contain both sites, and NR1/NR2B and NR1/NR2C, which contain only one.
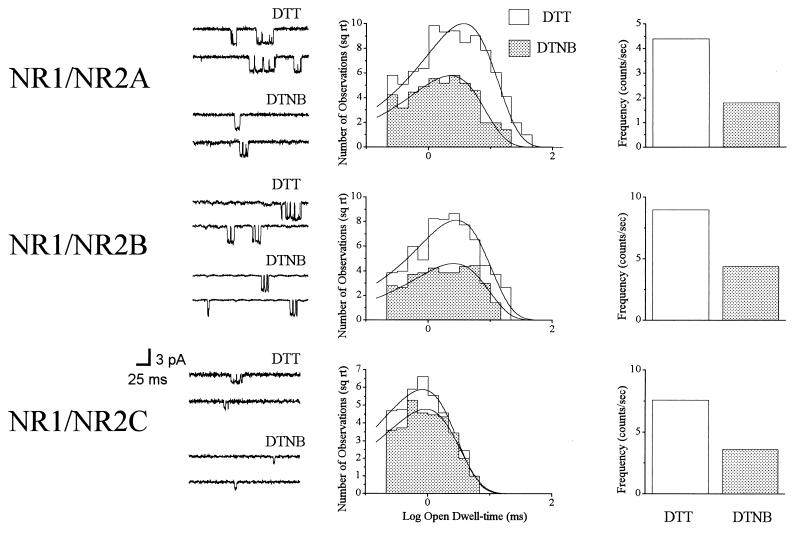
NMDA (10 μM)-activated channels were recorded from patches of cells transfected with either NR1/NR2A (n = 9), NR1/NR2B (n = 9), or NR1/NR2C (n = 4) during treatment with 1.0 mM DTT or 0.1 mM DTNB (Fig. 2). The single-channel amplitudes (−60 mV) of NR1/NR2A receptors were virtually identical under both redox conditions and were very similar to those obtained in the control patches (not shown). The mean time constants for the exponential functions fitting the open dwell-time distributions were 4.4 ± 0.4 ms in DTT (reduced) and 2.8 ± 0.2 ms in DTNB (oxidized; P < 0.0001, paired t test). The mean difference in the two time constants (ΔOTRDX) for all NR1/NR2A patches was 1.6 ± 0.2 ms. Additionally, the frequency of channel opening was 5.7- ± 3.4-fold greater (n-fold = x2/x1) in DTT than in DTNB, which, together with the changes in open time, produced an average 8.5- ± 3.9-fold increase in nPo.
Figure 2.
Redox properties of recombinant NMDA receptors. Representative NMDA (10 μM)-activated single channels obtained from outside-out patches excised from CHO cells transfected with NR1/NR2A (n = 9), NR1/NR2B (n = 9), and NR1/NR2C (n = 4). (Left) Representative events shown were recorded during continuous exposure to 1.0 mM DTT or 0.1 mM DTNB at −60 mV. The amplitudes of the events were not altered by the redox treatments for all receptor configurations (not shown) and were essentially identical to control channels. (Center) Open dwell-time histograms for these events were best-fit by single exponential functions; only the NR1/NR2A mean open dwell-time constants were significantly altered by the redox treatments. (Right) Frequency of channel opening was similarly altered by the redox treatments for all receptor configurations.
The mean amplitude of NR1/NR2B-containing channels also was unaltered by the redox treatments and very similar in treated and untreated patches (not shown). In addition, the frequency of channel opening was 2.0- ± 0.6-fold greater in DTT than DTNB. However, the difference in mean open dwell-time between reduced (3.0 ± 0.3 ms) and oxidized (2.7 ± 0.3) states was not statistically significant. The ΔOTRDX was 0.3 ± 0.2 ms for these channels. Hence, the increase in opening frequency alone could account for the observed 2.3- ± 0.6-fold increase in nPo in the reduced versus oxidized condition. Similar to NR1/NR2B receptors, redox agents did not affect the single-channel amplitude (not shown) or the mean open dwell-time of NR1/NR2C channels (0.7 ± 0.1 ms in DTT and 0.6 ± 0.1 ms in DTNB). The mean difference in the two time constants, ΔOTRDX, was 0.0 ± 0.1 ms for these receptors. Finally, the frequency of channel opening increased 2.2- ± 0.3-fold, and nPo 2.4- ± 0.2-fold, in the presence of DTT, when compared with DTNB in NR1/NR2C receptor channels.
Effects of CP101,606 on Recombinant Channel Function.
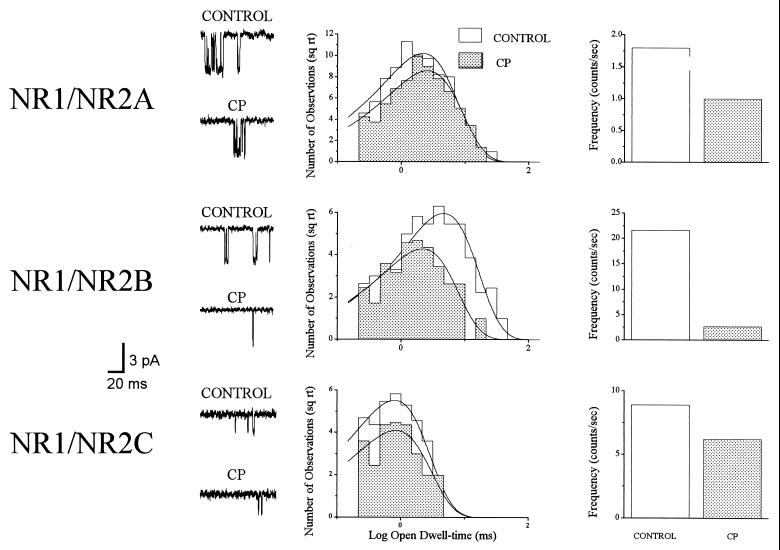
NMDA (10 μM)-activated channels were recorded in patches excised from cells transfected with either NR1/NR2A (n = 6), NR1/NR2B (n = 8), or NR1/NR2C (n = 3) before and after treatment with 1 μM CP (Fig. 3). Single-channel amplitudes (−60 mV) for all receptor configurations were unaffected by this antagonist (not shown). Furthermore, CP did not significantly alter the mean open-dwell time of NR1/NR2A receptors (2.7 ± 0.4 ms control, 2.7 ± 0.3 ms CP), but produced a 42.2 ± 7.7% decrease in the frequency of channel opening. The mean difference in open time constants between control and drug-treated conditions (ΔOTCP) for this receptor configuration was 0.0 ± 0.4 ms. Similarly, CP had no effect on the open dwell-time of NR1/NR2C channels (0.8 ± 0.1 ms control, 0.9 ± 0.0 ms CP), with a ΔOTCP of −0.1 ± 0.1 ms. This drug produced a 30.7 ± 5.5% decrease on NR1/NR2C frequency of channel opening. CP, however, produced a large reduction in the mean open dwell-time of NR1/NR2B channels (3.1 ± 0.5 ms control, 1.4 ± 0.2 ms drug, P < 0.01, paired t test), with a ΔOTCP of 1.7 ± 0.5 ms. In addition, this drug produced an 73.0 ± 7.4% decrease in frequency of channel opening, resulting in a 84.3 ± 6.1% decrease in NR1/NR2B nPo.
Figure 3.
CP is a selective NR1/NR2B antagonist. (Left) Representative 10 μM NMDA-activated events before and after treatment with 1 μM CP in patches excised from cells transfected with NR1/NR2A, NR1/NR2B, and NR1/NR2C. (Center) Open dwell-time histograms for the events evoked from these patches revealed that NR1/NR2A receptors are relatively unaffected by the presence of CP; although the frequency of channel opening of this channel (Right) is reduced by the antagonist. Similar results were obtained with NR1/NR2C channels. In contrast, the NR1/NR2B open time decreased to a large degree in the presence of CP. The frequency of channel opening for the NR1/NR2B channel also decreased substantially in the presence of this drug. Similar results were obtained in a total of six NR1/NR2A, eight NR1/NR2B, and three NR1/NR2C channels.
Putative Coassembly of NR2A/NR2B.
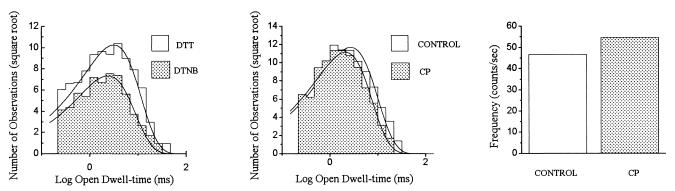
NMDA (10 μM)-induced unitary currents were obtained under control conditions and after treatment with 1 mM DTT, 0.1 mM DTNB, and 1 μM CP in 13 patches excised from cells transfected with NR1/NR2A/NR2B. The order of redox treatments was randomly varied among patches, but they always preceded the CP treatment. Channels from all these patches had approximately the same amplitude under all treatments (−3.4 ± 0.0 pA, −60 mV), and in all cases DTT treatment induced a 2.2- ± 0.4-fold increase in the frequency of channel opening, when compared with DTNB. Three of the 13 patches had events with properties similar to NR1/NR2A receptors, namely a significant (P < 0.05, paired t test) difference in mean open time between redox states and limited sensitivity to CP (not shown). Five patches had events that behaved very similarly to NR1/NR2B channels, in that the redox treatments produced little or no change in open time, whereas CP produced a decrease in both frequency of channel opening and open dwell-time (not shown). The remaining five patches had channels with properties that could not be easily categorized as either NR1/NR2A or NR1/NR2B (Table 1). Of these, three patches contained events with open dwell-times (3.1 ± 0.1 ms DTT, 2.6 ± 0.1 ms DTNB; ΔOTRDX = 0.5 ± 0.1 ms) not significantly affected by redox treatments (NR1/NR2B-like), but were relatively insensitive to CP block (Fig. 4). The mean open dwell-times were 2.7 ± 0.1 ms under control conditions and 2.7 ± 0.4 ms in CP (ΔOTCP = 0.0 ± 0.4 ms), similar to NR1/NR2A-containing channels. Furthermore, the frequency of channel opening in these three patches was only decreased 3.8 ± 19.6% by CP. A one-way ANOVA, followed by post-hoc comparison between means, revealed that the ΔOTRDX for this group of patches (0.5 ± 0.1 ms) was significantly different from the value shown earlier for NR1/NR2A-containing patches (P < 0.01), but not different from the NR1/NR2B value. Similarly, the ΔOTCP for the three patches (0.0 ± 0.4 ms), was not significantly different from the NR1/NR2A value, but different from the NR1/NR2B value (P < 0.05). The data strongly argues that activity in these patches results from channels assembled by both NR2A and NR2B. The remaining two patches had channels with properties that could not be easily grouped into any of the aforementioned categories.
Table 1.
Functional properties of recombinant NMDA receptors
| Proposed subunit composition | ΔOTRDX* | FRDX† | ΔOTCP‡ | FCP§ |
|---|---|---|---|---|
| NR1/NR2A | + | + | − | + |
| NR1/NR2B | − | + | + | + |
| NR1/NR2C | − | + | − | + |
| NR1/NR2A/NR2B | − | + | − | − |
+, significant difference between the two experimental conditions.
−, no significant difference between the two experimental conditions.
Difference in the open dwell-time constants of 10 μM NMDA-activated events recorded under reduced (1.0 mM DTT) and oxidized (0.1 mM DTNB) conditions.
Change in the frequency of NMDA channel opening between reduced and oxidized states.
Difference in the open dwell-time constants of 10 μM NMDA-induced events recorded under control conditions and in the presence of 1 μM CP.
Change in the frequency of channel opening in the absence and presence of CP.
Figure 4.
Putative coassembly of NR1/NR2A/NR2B. (Left) Open dwell-time histogram for events elicited with 10 μM NMDA in the presence of either 1 mM DTT or 0.1 mM DTNB revealed that the open dwell-times of these patches were unaffected by redox agents. Treatment of these patches with 1 μM CP did not alter the open dwell-time constants (Center) or the frequency of channel opening (Right). Similar results were obtained in a total of three channels from patches excised from cells transfected with NR1/NR2A/NR2B subunits.
DISCUSSION
Recombinant NMDA receptors containing either NR1/NR2A, NR1/NR2B, or NR1/NR2C subunit combinations have similar single-channel properties in different expression systems. The parameters obtained in the present study compare well with those obtained previously with HEK 293 cells and frog oocytes (21, 30). Surprisingly, the mean open time for recombinant receptors under control conditions is very similar to the values obtained after DTNB treatment. This suggests that the receptors in these patches are normally oxidized despite the fact that previous whole-cell studies revealed that a significant number of receptors in any given cell may lie in the reduced state (31). Therefore, the absence of unknown cellular factors, or perhaps even the temporary mechanical disruption of the membrane during patch formation, can lead to the oxidation of receptors that previously were reduced in the intact cell. This observation argues that the analysis of NMDA channel behavior must take into account that the receptor can lie in different redox states, which, in turn, could have pronounced repercussions in what is conceived as “normal” function, at least during steady-state measurements. For example, we have observed that Mg2+ and Zn2+ block, as well as current rectification properties, can change after redox treatments (32, 35).
The demonstrated presence of two redox modulatory sites in the NMDA receptor (33, 34) led us to evaluate whether each distinct site contributes differently to the overall properties of recombinant receptors. Sulfhydryl agents altered the frequency of channel opening of NR1/NR2A, NR1/NR2B, and NR1/NR2C receptors in a matter analogous to what is observed in native cortical receptors (32). Interestingly, redox agents affected the open dwell-time of NR1/NR2A channels, but not of NR1/NR2B- or NR1/NR2C-containing receptors, suggesting that the presence of the additional redox site on the NR2A subunit can be detected at the microscopic level. In vivo and in vitro studies in cortical neurons have shown that NR2A and NR2B are expressed together, and triplex heteromers (three different subunits arranged as a heteromer with unknown stoichiometry) containing NR1/NR2A/NR2B can be formed (10, 11, 20, 36, 37). In previous investigations (32), we observed that the open time of native receptors from cortical neurons was not substantially altered by sulfhydryl reagents, implying that these channels are not assembled by the combination of NR1 and NR2A alone. The present experiments also demonstrate that the novel drug CP more selectively blocks NR1/NR2B channels by substantially decreasing both their open time and frequency of opening. In contrast, this drug only decreased NR1/NR2A and NR1/NR2C frequency of channel opening, but to a lesser extent than NR1/NR2B channels. These actions of CP on NR1/NR2B-containing receptors are very similar to those produced by ifenprodil on NMDA receptors in hippocampal neurons in vitro (38).
Immunoprecipitation and functional studies have shown that multiple NR2 subunits can coassemble with NR1. Sheng et al. (10), Luo et al. (11), and Chazot et al. (9) were able to demonstrate that NR1 could be coimmunoprecipitated with both NR2A/NR2B or with NR2A/NR2C (but see ref. 39). Moreover, a functional assay led Wafford et al. (8) to conclude that NR1/NR2A/NR2C triplex-heteromers were preferentially assembled by the oocyte expression system when all three subunit mRNAs were coinjected. The present work shows the potential functional consequences of the assembly of three different NR subunits at the single channel level. The prominent features that characterized NR1/NR2A channels were a large shift in open time between redox states, plus a limited sensitivity to block by CP. On the other hand, the open time of NR1/NR2B channels was largely unaffected by redox conditions, whereas CP substantially decreased both the open time and the frequency of channel opening of these receptors. Of the 13 patches from cells transfected with all three subunits, eight had channels with properties that closely resembled the aforementioned profiles for NR1/NR2A or NR1/NR2B. Three of the remaining five patches yielded channels with a surprising combination of properties that could be explained by the formation of triplex-heteromeric receptors. Hence, the absence of redox sensitivity, vis-à-vis open time, and insensitivity to CP enabled us to group these patches into a novel category. Can we be certain that a mix of duplex-heteromeric receptors in a single patch cannot account for the appearance of this combination of pharmacological properties? The answer appears to be yes. These channels had redox properties identical to NR1/NR2B receptors, but were insensitive to CP block. No combination of duplex-heteromeric receptors, assuming that the properties of NR1/NR2A and NR1/NR2B duplex-heteromeric receptors, such as subunit order, do not change when the three different subunits are cotransfected, could possibly account for this behavior, as we would have expected some intermediate redox-dependent changes in open time, or a certain degree of block by CP.
These investigations provide evidence that the presence of both NR2A and NR2B in a single receptor imparts this protein a different set of pharmacological attributes when compared with receptors containing a single type of these subunits. Hence, the presence of NR2B somehow can abolish the effects of redox agents on channel open time, whereas the presence of NR2A can decrease the sensitivity of the receptor to ifenprodil analogues. Approaches similar to the one taken here may prove useful in functionally assessing the subunit composition of NMDA receptors in neurons.
Acknowledgments
We thank K. Hartnett and W. Potthoff for technical assistance, S. Nakanishi, P. Seeburg, and M. Chalfie for plasmids, W.F. White for CP, and J. Dilmore, S. Antonov, Y. Li-Smerin, I. Reynolds, and J. Horn for suggestions. This work was supported by National Institutes of Health Grant NS29365.
ABBREVIATIONS
- NMDA
N-methyl-d-aspartate
- NR
NMDA receptor subunit
- DTNB
5,5′-dithio-bis[2-nitrobenzoic acid]
- CHO
Chinese hamster ovary
- CP
CP101,606
- nPo
open channel probability
Note Added in Proof
A very recent study (40) has suggested that the putative redox site on the NR2A subunit actually represents a high-affinity binding site for Zn2+. In a series of preliminary studies in our laboratory we have observed that nonreducing metal chelating agents, such as N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN, 10 μM), do not affect NMDA-induced currents mediated by NR1/NR2A receptors expressed in CHO cells. In contrast, DTT always potentiates NR1/NR2A-mediated currents, even when NR1 contains a cysteine for serine substitution at position 744, which abolishes the redox site on this subunit (33). This suggests that a complex mechanism is likely responsible for the modification of the NR2A subunit by reducing agents.
References
- 1.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Nature (London) 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 2.Zukin R S, Bennett M V. Trends Neurosci. 1995;18:306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda K, Nagasawa H, Mori H, Araki K, Sakimura K, Watanabe M, Inoue Y, Mishina M. FEBS Lett. 1992;313:34–38. doi: 10.1016/0014-5793(92)81178-o. [DOI] [PubMed] [Google Scholar]
- 4.Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Nature (London) 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- 5.Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M. Nature (London) 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- 6.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg P H. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 7.Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- 8.Wafford K A, Bain C J, Le Bourdelles B, Whiting P J, Kemp J A. NeuroReport. 1993;4:1347–1349. doi: 10.1097/00001756-199309150-00015. [DOI] [PubMed] [Google Scholar]
- 9.Chazot P L, Coleman S K, Cik M. J Biol Chem. 1994;269:24403–24409. [PubMed] [Google Scholar]
- 10.Sheng M, Cummings J, Roldan L A, Jan Y N, Jan L Y. Nature (London) 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Wang Y, Yasuda R P, Dunah A W, Wolfe B B. Mol Pharmacol. 1997;51:79–86. doi: 10.1124/mol.51.1.79. [DOI] [PubMed] [Google Scholar]
- 12.Boeckman F A, Aizenman E. Neurosci Lett. 1994;173:189–192. doi: 10.1016/0304-3940(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 13.McIlhinney R A J, Molnar E, Atack J R, Whiting P J. Neuroscience. 1996;70:989–997. doi: 10.1016/0306-4522(95)00419-x. [DOI] [PubMed] [Google Scholar]
- 14.Bèhè P, Stern P, Wyllie D J, Nassar M, Schoepfer R, Colquhoun D. Proc R Soc London B. 1995;262:205–213. doi: 10.1098/rspb.1995.0197. [DOI] [PubMed] [Google Scholar]
- 15.Premkumar L S, Auerbach A. Soc Neurosci Abstr. 1996;22:593. [Google Scholar]
- 16.Williams K. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- 17.Williams K, Zappia A M, Pritchett D B, Shen Y M, Molinoff P B. Mol Pharmacol. 1994;45:803–809. [PubMed] [Google Scholar]
- 18.Lynch D R, Gallagher M J. J Pharmacol Exp Therap. 1996;279:154–161. [PubMed] [Google Scholar]
- 19.Gallagher M J, Huang H, Pritchett D B, Lynch D R. J Biol Chem. 1996;271:9603–9611. doi: 10.1074/jbc.271.16.9603. [DOI] [PubMed] [Google Scholar]
- 20.Monyer H, Burnashev N, Laurie D J, Sakmann B, Seeburg P H. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 21.Stern P, Bèhè P, Schoepfer R, Colquhoun D. Proc R Soc London B. 1992;250:271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Zheng X, Paupard M C, Wang A P, Santchi L, Friedman L K, Zukin R S, Bennett M V L. Proc Natl Acad Sci USA. 1994;91:10883–10887. doi: 10.1073/pnas.91.23.10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnashev N, Zhou Z, Neher E, Sakmann B. J Physiol. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuner T, Schoepfer R. J Neurosci. 1996;16:3549–3558. doi: 10.1523/JNEUROSCI.16-11-03549.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boeckman F A, Aizenman E. J Pharmacol Exp Therap. 1996;279:515–523. [PubMed] [Google Scholar]
- 26.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Science. 1994;262:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 27.Cik M, Chazot P L, Stephenson F A. Biochem J. 1993;296:877–883. doi: 10.1042/bj2960877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chenard B L, Bordner J, Butler T W, Chambers L K, Collins M A, De Costa D L, Ducat M F, Dumont M L, Fox C B, Mena E E, Menniti F S, Nielson J, Pagnozzi M J, Richter K E J, Ronau R T, Shalaby I A, Stemple J Z, White W F. J Med Chem. 1995;38:3138–3145. doi: 10.1021/jm00016a017. [DOI] [PubMed] [Google Scholar]
- 29.Ascher P, Nowak L. J Physiol. 1988;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern P, Cik M, Colquhoun D, Stephenson F A. J Physiol. 1994;476:391–397. doi: 10.1113/jphysiol.1994.sp020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aizenman E, Lipton S A, Loring R E. Neuron. 1989;2:1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
- 32.Tang L-H, Aizenman E. J Physiol. 1993;465:303–323. doi: 10.1113/jphysiol.1993.sp019678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan J M, Traynelis S F, Chen H V, Escobar W, Heinemann S F, Lipton S A. Neuron. 1994;13:929–936. doi: 10.1016/0896-6273(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 34.Köhr G, Eckardt S, Lüddens H, Monyer H, Seeburg P H. Neuron. 1994;12:1031–1040. doi: 10.1016/0896-6273(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 35.Tang L-H, Aizenman E. Mol Pharmacol. 1993;44:473–478. [PubMed] [Google Scholar]
- 36.Zhong J, Russell S L, Pritchett D B, Molinoff P B, Williams W. Mol Pharmacol. 1994;45:846–853. [PubMed] [Google Scholar]
- 37.Zhong J, Carrozza D P, Williams K, Pritchett D B, Molinoff P B. J Neurochem. 1995;64:531–539. doi: 10.1046/j.1471-4159.1995.64020531.x. [DOI] [PubMed] [Google Scholar]
- 38.Legendre P, Westbrook G L. Mol Pharmacol. 1991;40:289–298. [PubMed] [Google Scholar]
- 39.Blahos J, II, Wenthold R J. J Biol Chem. 1996;271:15669–15674. doi: 10.1074/jbc.271.26.15669. [DOI] [PubMed] [Google Scholar]
- 40.Paoletti P, Ascher P, Neyton J. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]