Abstract
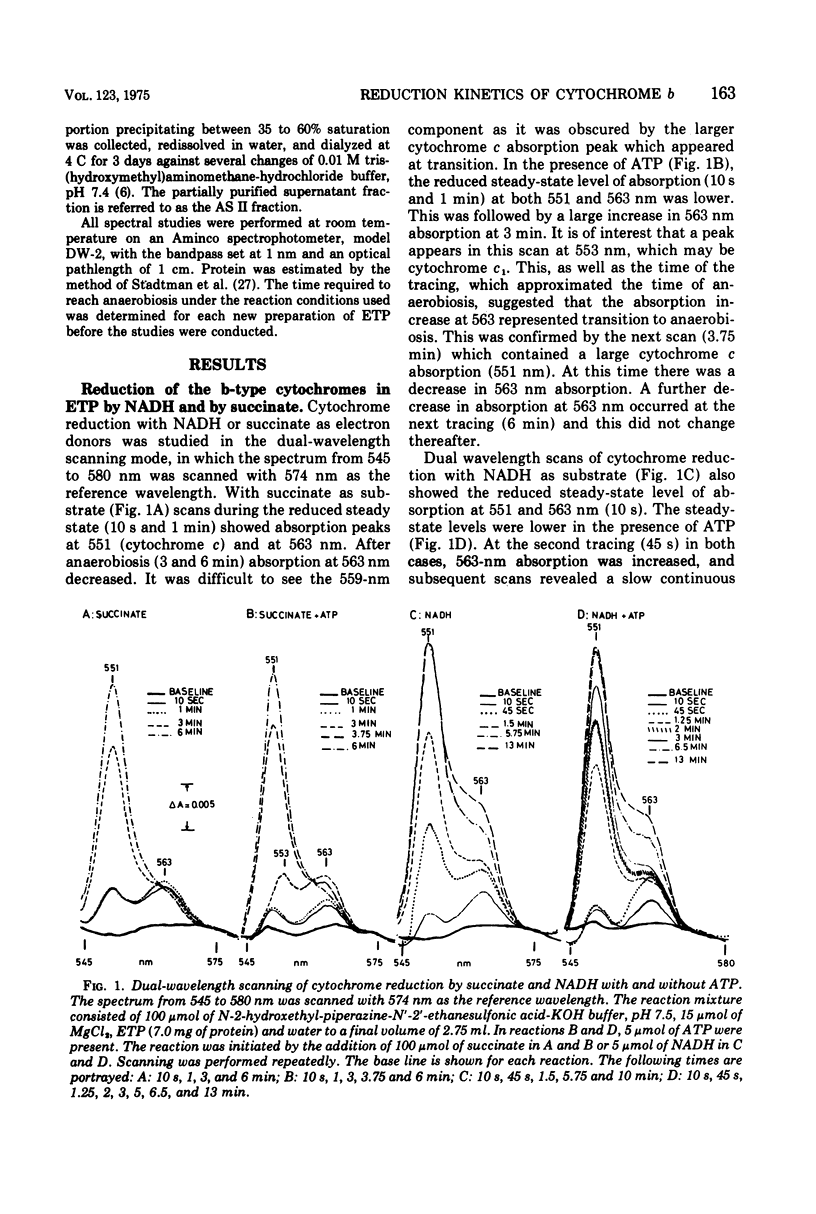
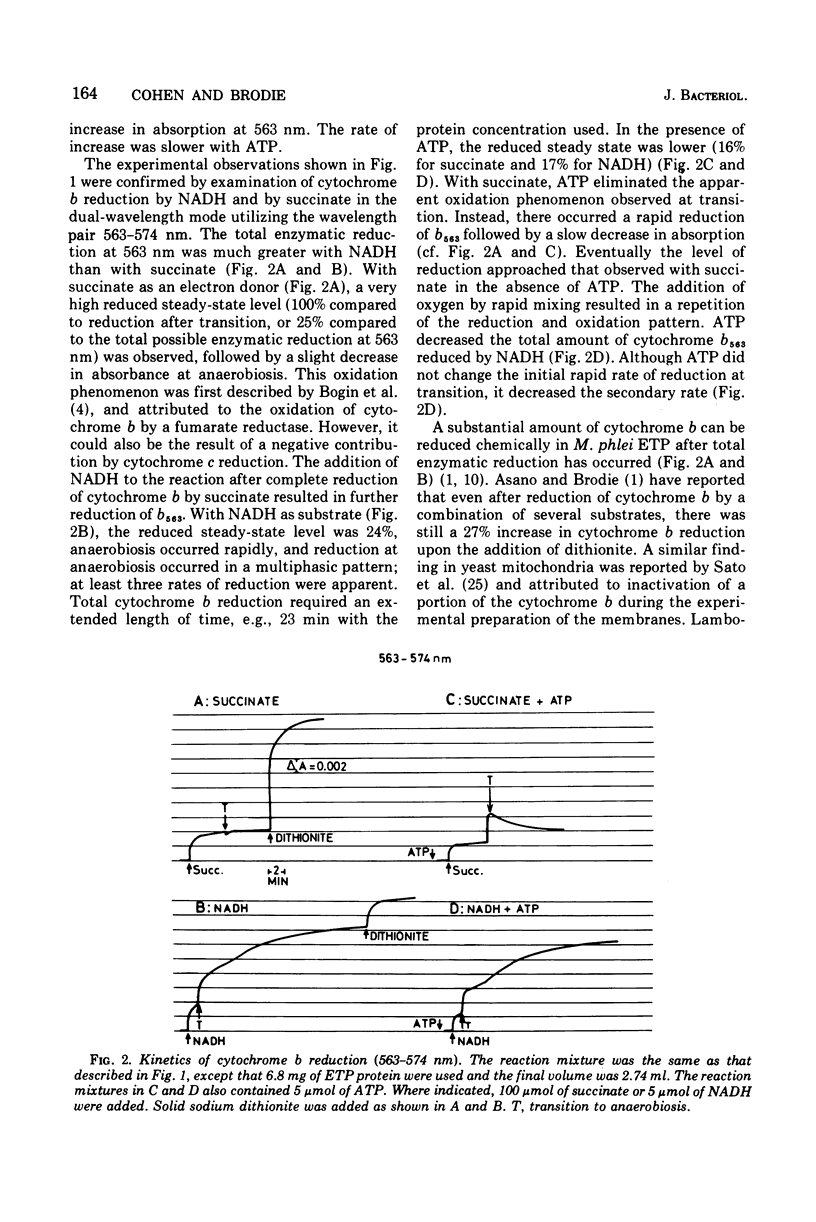
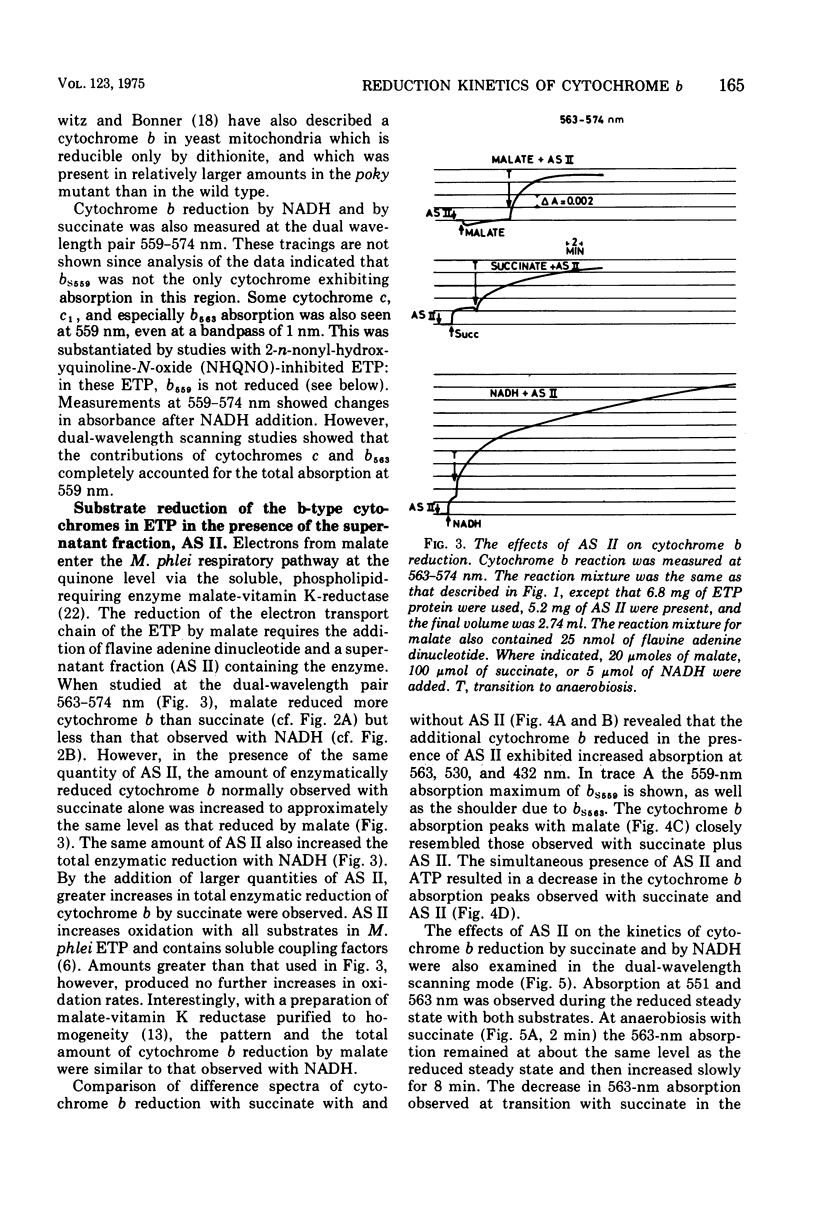
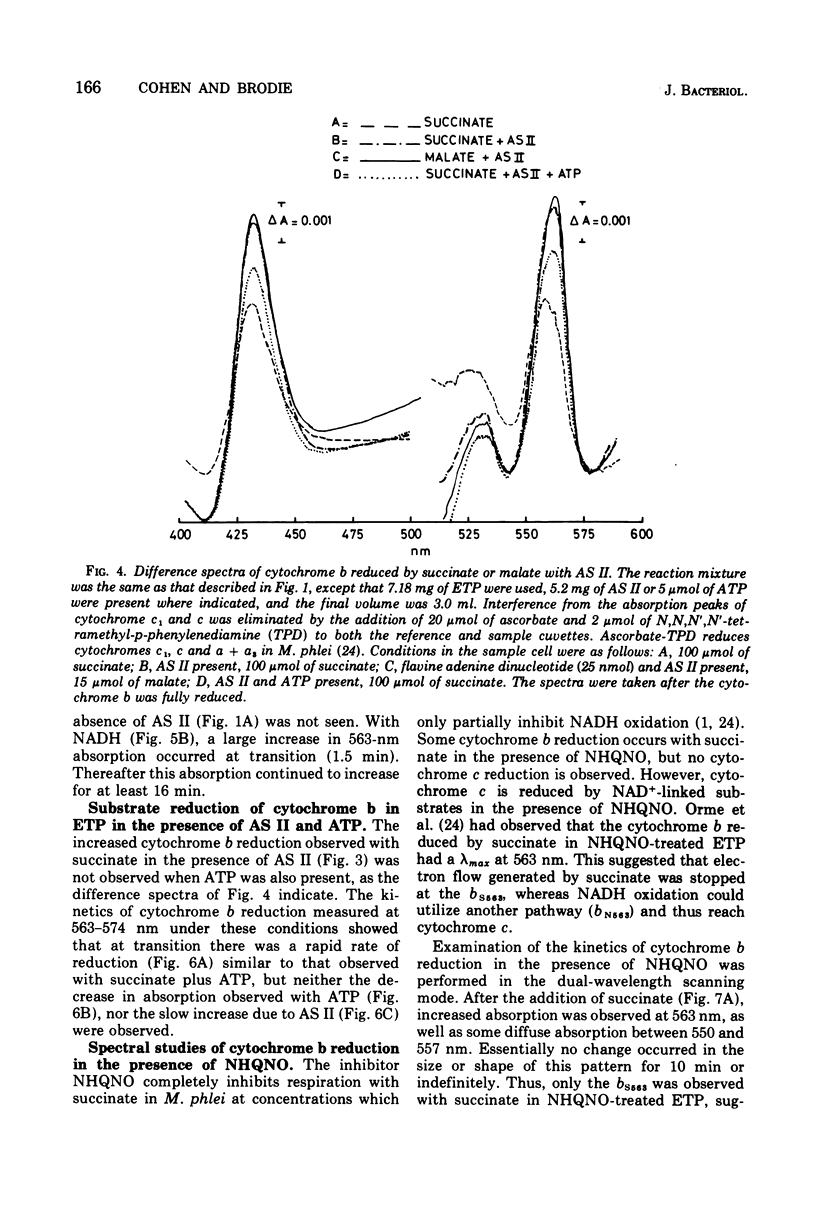
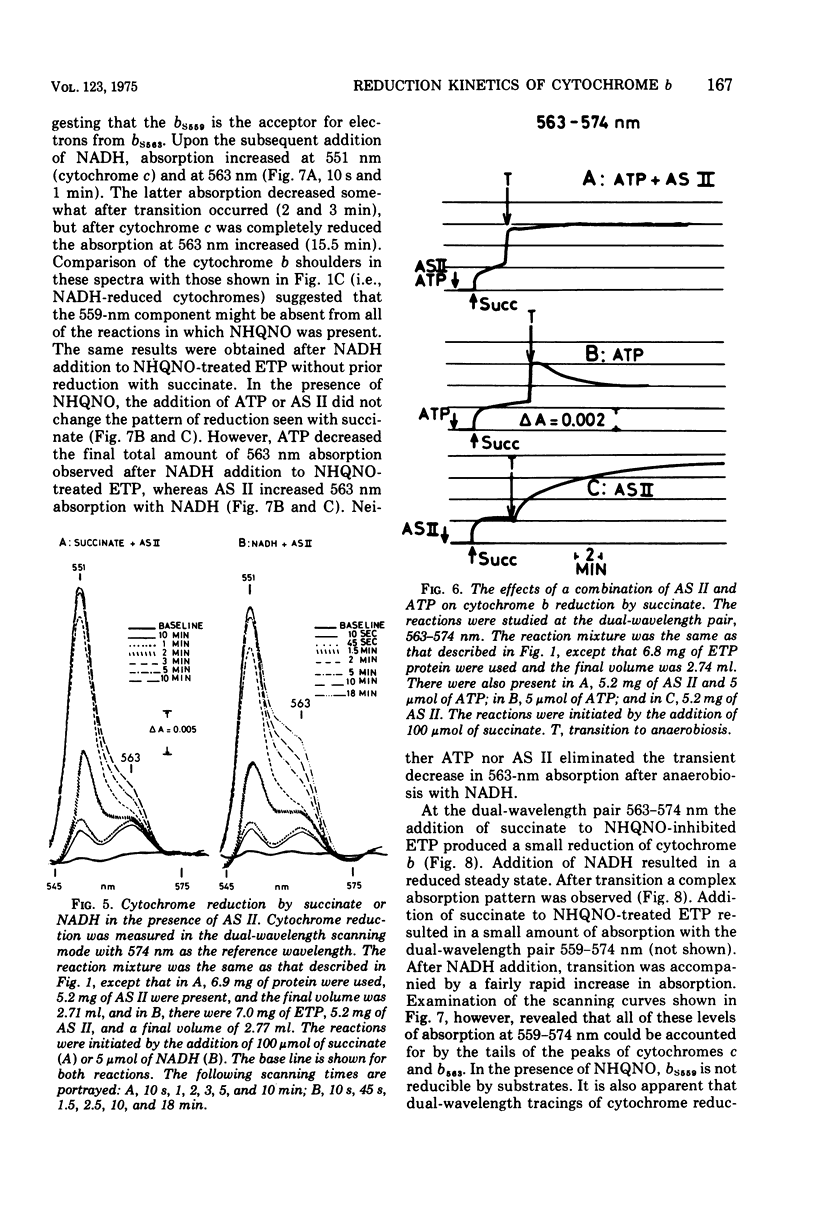
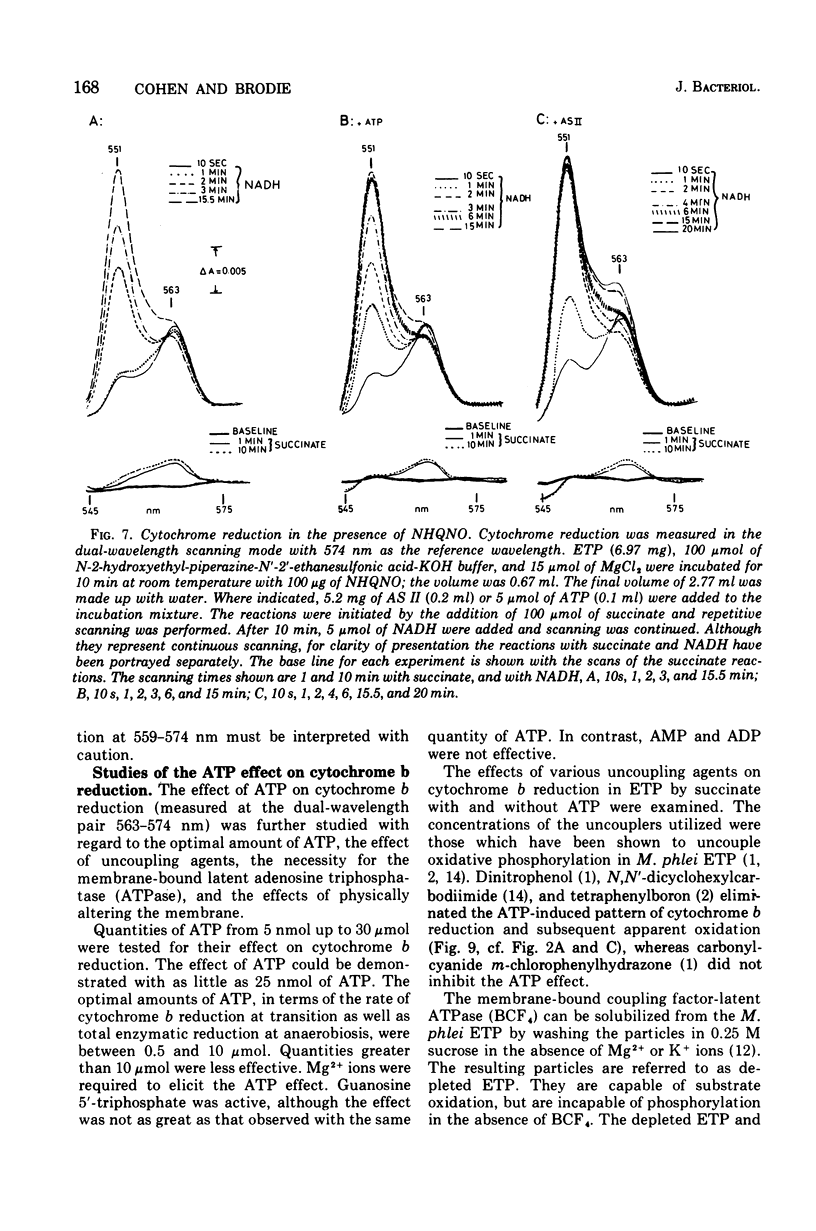
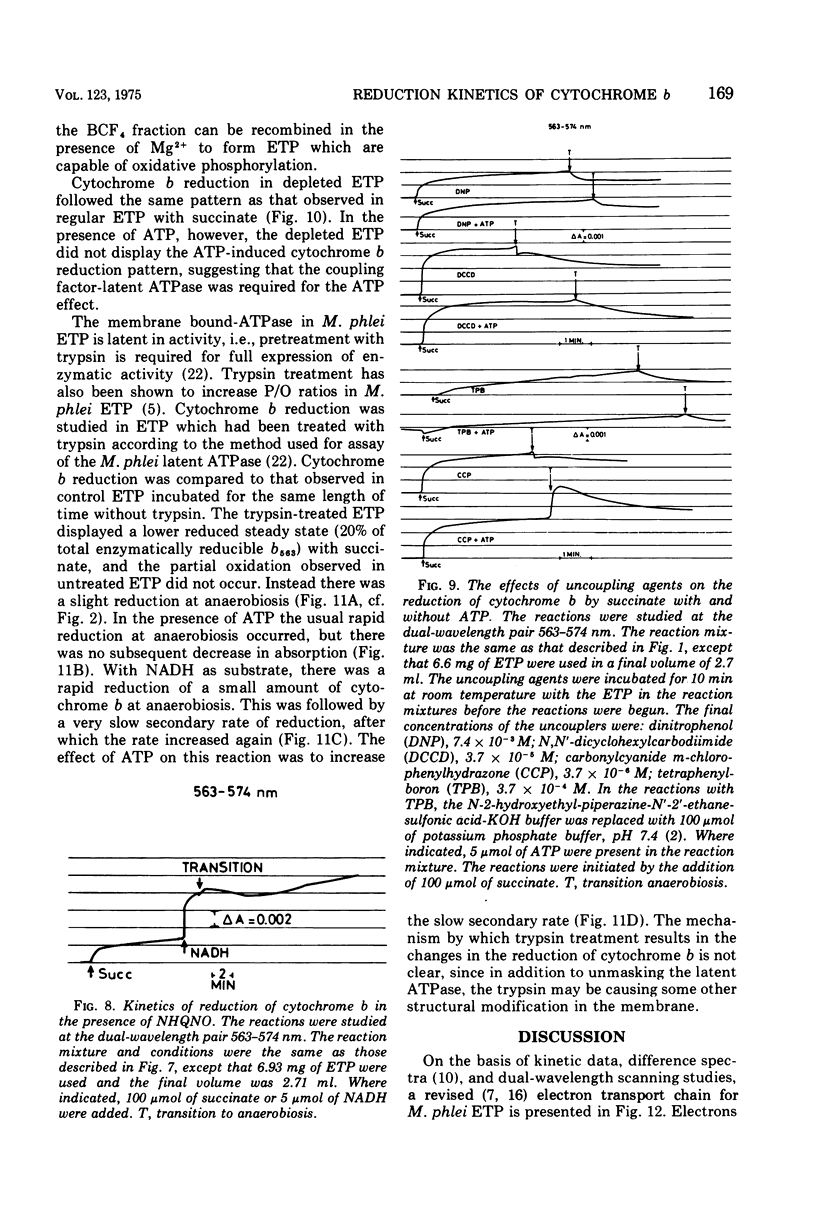
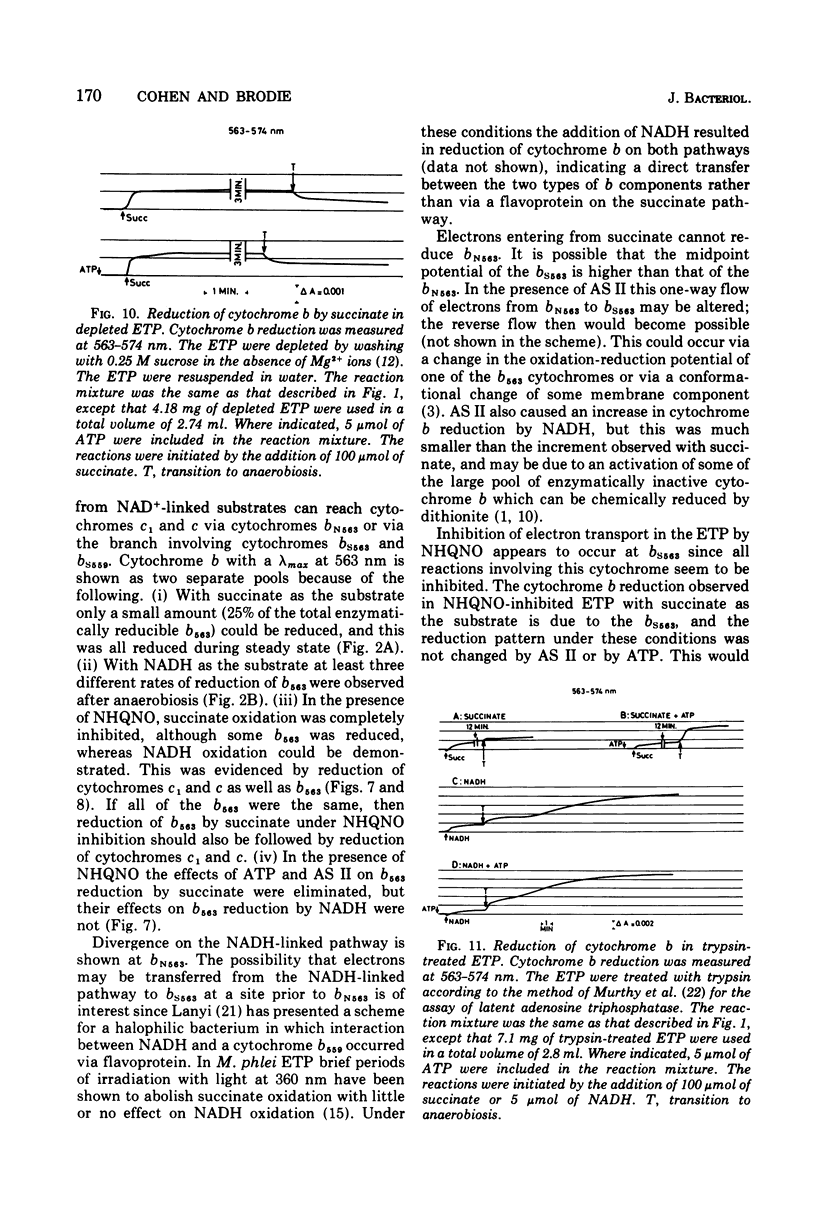
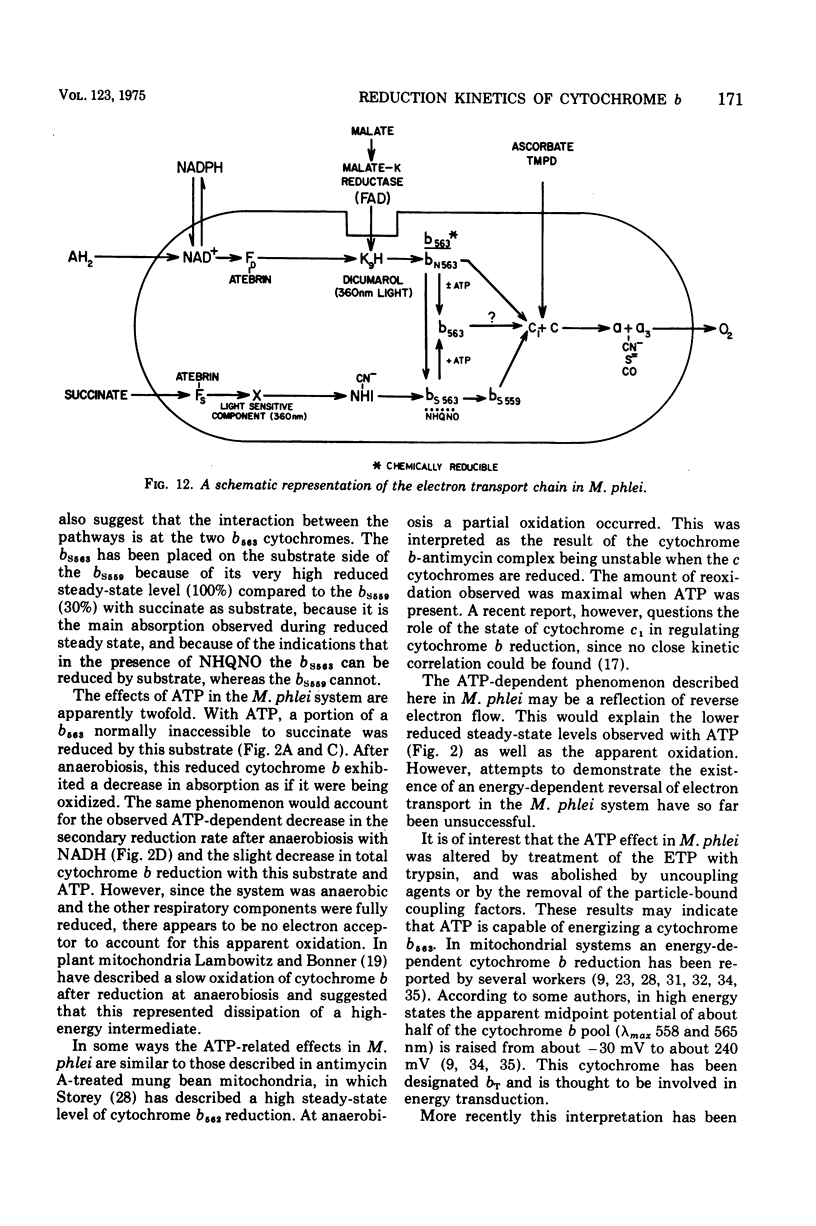
The kinetics of reduction of the b-type cytochromes in the electron transport particles (ETP) from Mycobacterium phlei were studied with nicotinamide adenine dinucleotide, reduced form (NADH) or succinate as electron donors. There appeared to be three active cytochromes b in the ETP,bS563 and bS559, which were reducible by either substrate, and bN563, which was reducible by NADH but not by succinate. In the presence of adenosine 5'-triphosphate, a substantial increase in b563 reduction was observed with succinate at anaerobiosis. This was followed by a decrease in absorption. Adenosine 5'-triphosphate did not effect an increase in cytochrome b563 reduction at transition with NADH, but the occurrence of a secondary decrease in absorption was reflected in a decrease in total enzymatic reduction. The adenosine 5'-triphosphate effect was altered in trypsin-treated ETP, and abolished by uncoupling agents or by removal of the coupling factor-latent adenosine triphosphatase. In the presence of a supernatant fraction obtained during the preparation of the ETP, b563 reduction with succinate was greatly increased. A smaller increase was observed with NADH. Cytochrome b reduction was also studied in ETP inhibited by 2-n-nonylhydroxyquinoline-N-oxide, which appears to inhibit at bS563. On the basis of these data the interrelationships among the b-type cytochromes can be described in relation to the M. phlei electron transport chain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASANO A., BRODIE A. F. OXIDATIVE PHOSPHORYLATION IN FRACTIONATED BACTERIAL SYSTEMS. XIV. RESPIRATORY CHAINS OF MYCOBACTERIUM PHLEI. J Biol Chem. 1964 Dec;239:4280–4291. [PubMed] [Google Scholar]
- Asano A., Cohen N. S., Baker R. F., Brodie A. F. Orientation of the cell membrane in ghosts and electron transport particles of Mycobacterium phlei. J Biol Chem. 1973 May 25;248(10):3386–3397. [PubMed] [Google Scholar]
- Asano A., Hirata H., Brodie A. F. A factor(s) required for activation of oxidative phosphorylation in protoplast ghosts of Mycobacterium phlei. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1340–1346. doi: 10.1016/s0006-291x(72)80122-0. [DOI] [PubMed] [Google Scholar]
- BRODIE A. F. Oxidative phosphorylation in fractionated bacterial systems. I. Role of soluble factors. J Biol Chem. 1959 Feb;234(2):398–404. [PubMed] [Google Scholar]
- Bogin E., Higash T., Brodie A. F. Exogenous NADH oxidation and particulate fumarate reductase in Mycobacterium phlei. Arch Biochem Biophys. 1969 Jan;129(1):211–220. doi: 10.1016/0003-9861(69)90168-4. [DOI] [PubMed] [Google Scholar]
- Bogin E., Higashi T., Brodie A. F. The effect of trypsin and heat treatment on oxidative phosphorylation in Mycobacterium phlei. Biochem Biophys Res Commun. 1970 Nov 25;41(4):995–1001. doi: 10.1016/0006-291x(70)90183-x. [DOI] [PubMed] [Google Scholar]
- Brodie A. F., Adelson J. Respiratory Chains and Sites of Coupled Phosphorylation. Science. 1965 Jul 16;149(3681):265–269. doi: 10.1126/science.149.3681.265. [DOI] [PubMed] [Google Scholar]
- CHANCE B. The kinetics and inhibition of cytochrome components of the succinic oxidase system. III. Cytochrome b. J Biol Chem. 1958 Nov;233(5):1223–1229. [PubMed] [Google Scholar]
- Chance B., Wilson D. F., Dutton P. L., Erecińska M. Energy-coupling mechanisms in mitochondria: kinetic, spectroscopic, and thermodynamic properties of an energy-transducing form of cytochrome b. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1175–1182. doi: 10.1073/pnas.66.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N. S., Bogin E., Higashi T., Brodie A. F. Multiple cytochromes b in Mycobacterium phlei. Biochem Biophys Res Commun. 1973 Sep 18;54(2):800–807. doi: 10.1016/0006-291x(73)91495-2. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y., Poff K. L., Butler W. L. The b-type cytochromes of beef heart mitochondria. Biochem Biophys Res Commun. 1972 Mar 24;46(6):1984–1990. doi: 10.1016/0006-291x(72)90748-6. [DOI] [PubMed] [Google Scholar]
- Higashi T., Bogin E., Brodie A. F. Separation of a factor indispensable for coupled phosphorylation from the particulate fraction of Mycobacterium phlei. J Biol Chem. 1969 Jan 25;244(2):500–502. [PubMed] [Google Scholar]
- Kalra V. K., Brodie A. F. Effect of N,N'-dicyclohexylcarbodiimide (DCCD) on electron transport particles of Mycobacterium phlei. Arch Biochem Biophys. 1971 Dec;147(2):653–659. doi: 10.1016/0003-9861(71)90424-3. [DOI] [PubMed] [Google Scholar]
- Kurup C. K., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. XXII. The effect of near ultraviolet irradiation on the succinate oxidase pathway of Mycobacterium phlei. J Biol Chem. 1966 Sep 10;241(17):4016–4022. [PubMed] [Google Scholar]
- Kurup C. K., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. XXIX. The involvement of nonheme iron in the respiratory pathways of Mycobacterium phlei. J Biol Chem. 1967 Dec 25;242(24):5830–5837. [PubMed] [Google Scholar]
- Lambowitz A. M., Bonner W. D., Jr The b-cytochromes of plant mitochondria. A spectrophotometric and potentiometric study. J Biol Chem. 1974 Apr 25;249(8):2428–2440. [PubMed] [Google Scholar]
- Lambowitz A. M., Bonner W. D., Jr The effect of antimycin on the b-cytochromes of plant mitochondria. Oxidation-reduction behavior of cytochrome b-566. Biochem Biophys Res Commun. 1973 Jun 8;52(3):703–711. doi: 10.1016/0006-291x(73)90994-7. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Bonner W. D., Jr The mitochondrial beta-cytochromes of the wild type and poky strains of Neurospora crassa. Evidence for a component reduced only by dithionite. J Biol Chem. 1974 May 10;249(9):2886–2890. [PubMed] [Google Scholar]
- Lambowitz A. M., Bonner W. D., Jr, Wikström M. K. On the lack of ATP-induced midpoint potential shift for cytochrome b-566 in plant mitochondria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1183–1187. doi: 10.1073/pnas.71.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. II. Salt dependence of reduced diphosphopyridine nucleotide oxidase. J Biol Chem. 1969 Jun 10;244(11):2864–2869. [PubMed] [Google Scholar]
- Murthy P. S., Bogin E., Higashi T., Brodie A. F. Properties of the soluble malate-vitamin K reductase and associated phosphorylation. J Biol Chem. 1969 Jun 25;244(12):3117–3124. [PubMed] [Google Scholar]
- Norling B., Nelson B. D., Nordenbrand K., Ernster L. Evidence for the occurrence in submitochondrial particles of a dual respiratory chain containing different forms of cytochrome b. Biochim Biophys Acta. 1972 Jul 12;275(1):18–32. doi: 10.1016/0005-2728(72)90021-7. [DOI] [PubMed] [Google Scholar]
- Orme T. W., Revsin B., Brodie A. F. Phosphorylation linked to ascorbate oxidation in Mycobacterium phlei. Arch Biochem Biophys. 1969 Oct;134(1):172–179. doi: 10.1016/0003-9861(69)90263-x. [DOI] [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]
- Sato N., Onishi T., Chance B. Spectral study of b cytochromes in yeast mitochondria and intact cells. Biochim Biophys Acta. 1972 Sep 20;275(3):288–297. doi: 10.1016/0005-2728(72)90209-5. [DOI] [PubMed] [Google Scholar]
- Slater E. C., Lee C. P., Berden J. A., Wegdam H. J. High-energy forms of cytochrome b. Nature. 1970 Jun 27;226(5252):1248–1249. doi: 10.1038/2261248a0. [DOI] [PubMed] [Google Scholar]
- Storey B. I. The respiratory chain of plant mitochondria. XV. Equilibration of cytochromes C549, b553, b557 and ubiquinone in Mung bean mitochondria: placement of cytochrome b 557 and estimation of the midpoint potential of ubiquinone. Biochim Biophys Acta. 1973 Apr 5;292(3):592–603. doi: 10.1016/0005-2728(73)90007-8. [DOI] [PubMed] [Google Scholar]
- Storey B. T., Lee C. P. Circular dichroism of cytochrome oxidase, cytochrome b 566 , and cytochrome c in beef heart mitochondrial membrane fragments. Biochim Biophys Acta. 1973 Apr 5;292(3):554–565. doi: 10.1016/0005-2728(73)90004-2. [DOI] [PubMed] [Google Scholar]
- Storey B. T. The respiratory chain of plant mitochondria. 13. Redox state changes of cytochrome b 562 in mung bean seedling mitochondria treated with antimycin A. Biochim Biophys Acta. 1972 Apr 20;267(1):48–64. doi: 10.1016/0005-2728(72)90137-5. [DOI] [PubMed] [Google Scholar]
- Wegdam H. J., Berden J. A., Slater E. C. High-energy forms of cytochrome b. II. The effect of ATP and antimycin on cytochrome b in intact mitochondria. Biochim Biophys Acta. 1970 Dec 8;223(2):365–373. doi: 10.1016/0005-2728(70)90193-3. [DOI] [PubMed] [Google Scholar]
- Wikström M. K. Properties of three cytochrome b-like species in mitochondria and submitochondrial particles. Biochim Biophys Acta. 1971 Dec 7;253(2):332–345. doi: 10.1016/0005-2728(71)90037-5. [DOI] [PubMed] [Google Scholar]
- Wikström M. K. The different cytochrome b components in the respiratory chain of animal mitochondria and their role in electron transport and energy conservation. Biochim Biophys Acta. 1973 Dec 7;301(2):155–193. doi: 10.1016/0304-4173(73)90003-7. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Dutton P. L. Energy dependent changes in the oxidation-reduction potential of cytochrome b. Biochem Biophys Res Commun. 1970 Apr 8;39(1):59–64. doi: 10.1016/0006-291x(70)90757-6. [DOI] [PubMed] [Google Scholar]
- Yu C. A., Yu L., King T. E. Spectral evidence of multiple cytochromes b present in succinate: cytochrome c reductase. Biochim Biophys Acta. 1972 May 25;267(2):300–308. doi: 10.1016/0005-2728(72)90118-1. [DOI] [PubMed] [Google Scholar]



