Abstract
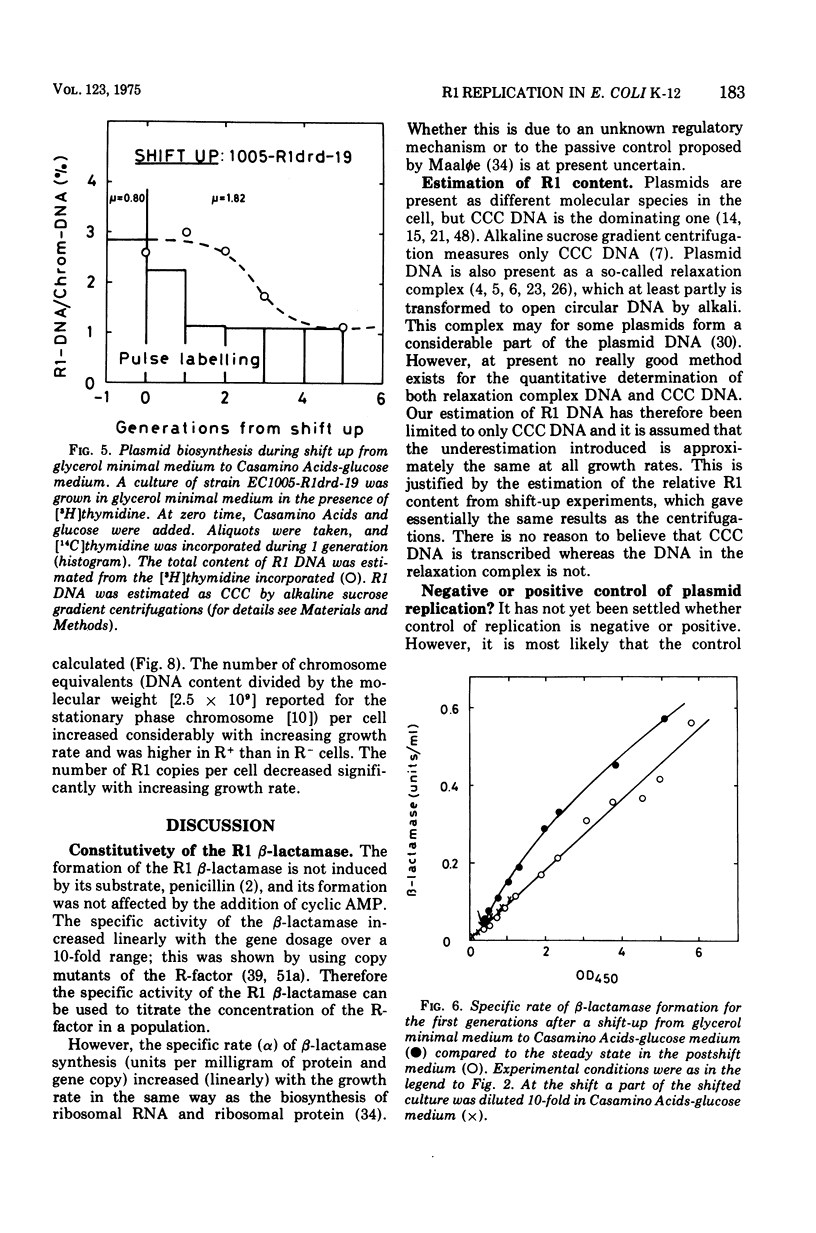
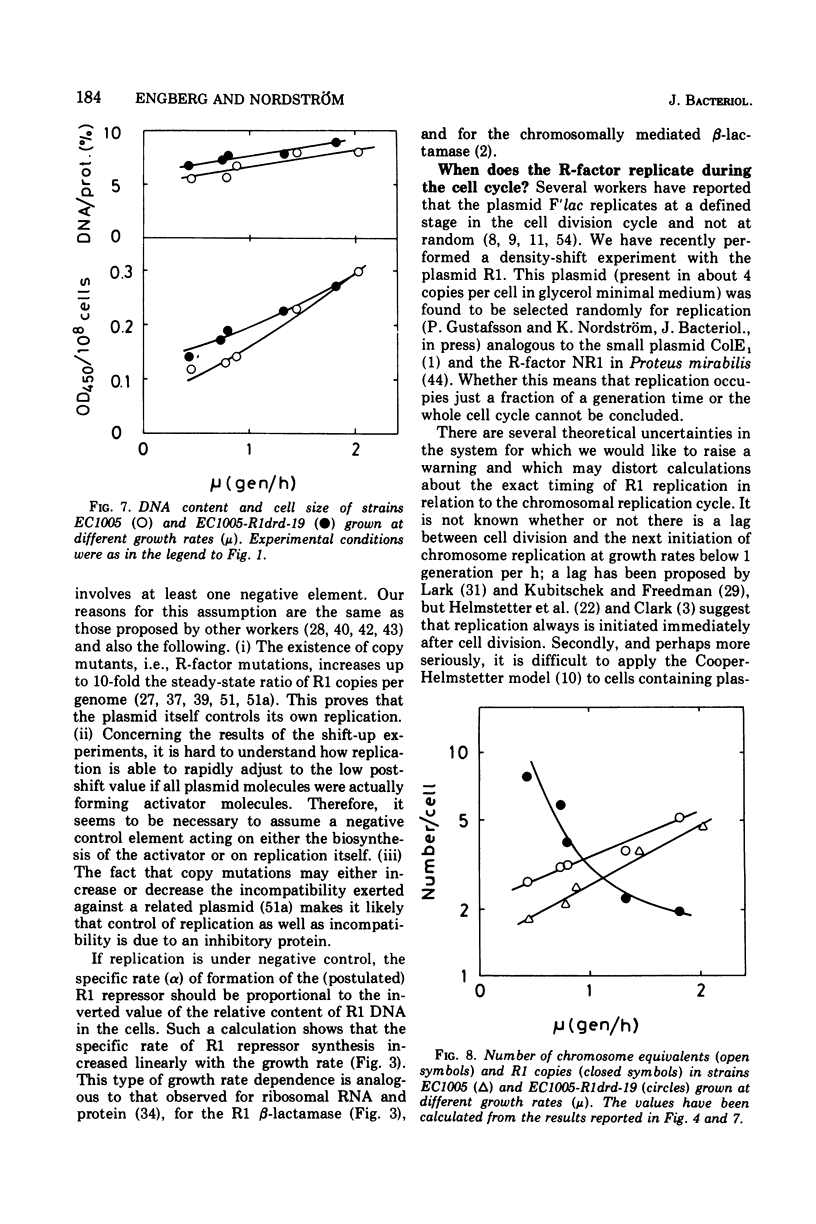
The R-factor R1drd-19 mediates resistance to beta-lactam antibiotics via a beta-lactamase. A strain of Escherichia coli K-12 carrying R1drd-19 was grown at different growth rates by using different carbon sources. The specific rate of production of the R1 beta-lactamase increased linearly with the growth rate and with the gene dosage. The content of R1 deoxyribonucleic acid was estimated by alkaline sucrose gradient centrifugation and by analysis of the specific rate of beta-lactamase synthesis in nutritional shift-up experiments and was found to decrease fivefold when the growth rate was increased from 0.4 to 1.8 doublings per h. The number of R1 molecules per cell decreased from six to two in the same growth range. The presence of the plasmid affected the mean cell size significantly; at a growth rate of 0.4 doublings per h the R-+ cells were on the average 50% bigger than the R-minus cells, whereas the effect was less than 10% at a growth rate of 1.8 doublings per h. Several reports in the leterature state that the initiation mass of chromosome replication is constant. In this paper it is shown that the initiation mass of R1 replication is proportional to the growth rate. Thus, the replication of the plasmid R1 and of the chromosome are independently regulated processes. It is argued that plasmid replication is under negative control.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazaral M., Helinski D. R. Replication of a bacterial plasmid and an episome in Escherichia coli. Biochemistry. 1970 Jan 20;9(2):399–406. doi: 10.1021/bi00804a029. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K., Boman H. G. Resistance of Escherichia coli to penicillins. V. Physiological comparison of two isogenic strains, one with chromosomally and one with episomally mediated ampicillin resistance. J Bacteriol. 1968 Aug;96(2):438–446. doi: 10.1128/jb.96.2.438-446.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J. The regulation of DNA replication and cell division in E. coli B-r. Cold Spring Harb Symp Quant Biol. 1968;33:823–838. doi: 10.1101/sqb.1968.033.01.094. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. E. Existence of the colicinogenic factor-sex factor ColI-b-P9 as a supercoiled circular DNA-protein relaxation complex. Biochem Biophys Res Commun. 1970 Oct 9;41(1):150–156. doi: 10.1016/0006-291x(70)90481-x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Evidence for the existence of the colicinogenic factors E2 and E3 as supercoiled circular DNA-protein relaxation complexes. Biochem Biophys Res Commun. 1970 Aug 11;40(3):608–613. doi: 10.1016/0006-291x(70)90947-2. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Pritchard R. H. Relationship between chromosome replication and F'lac episome replication in Escherichia coli. J Mol Biol. 1973 Jun 25;78(1):143–155. doi: 10.1016/0022-2836(73)90434-8. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Cooper S. Relationship of Flac replication and chromosome replication. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2706–2710. doi: 10.1073/pnas.69.9.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. B., Helmstetter C. E. Control of F'lac replication in Escherichia coli B-r. J Bacteriol. 1973 Apr;114(1):294–299. doi: 10.1128/jb.114.1.294-299.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968 Sep 7;219(5158):1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- Falkow S., Tompkins L. S., Silver R. P., Guerry P., Le Blanc D. J. The problems of drug-resistant pathogenic bacteria. The replication of R-factor DNA in Escherichia coli K-12 following conjugation. Ann N Y Acad Sci. 1971 Jun 11;182:153–171. doi: 10.1111/j.1749-6632.1971.tb30654.x. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Studies with Escherichia coli sex factors. Cold Spring Harb Symp Quant Biol. 1968;33:425–434. doi: 10.1101/sqb.1968.033.01.049. [DOI] [PubMed] [Google Scholar]
- Goebel W. Replication of the DNA of the colicinogenic factor E 1 (Col E 1 ) at the restrictive temperature in a DNA replication mutant thermosensitive for DNA polymerase. 3. Nat New Biol. 1972 May 17;237(72):67–70. doi: 10.1038/newbio237067a0. [DOI] [PubMed] [Google Scholar]
- Goebel W. The influence of DNA A and DNA C mutations on the initiation of plasmid DNA replication. Biochem Biophys Res Commun. 1973 Apr 16;51(4):1000–1007. doi: 10.1016/0006-291x(73)90026-0. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski D. R. Plasmid determined resistance to antibiotics: molecular properties of R factors. Annu Rev Microbiol. 1973;27:437–470. doi: 10.1146/annurev.mi.27.100173.002253. [DOI] [PubMed] [Google Scholar]
- Helmstetter C., Cooper S., Pierucci O., Revelas E. On the bacterial life sequence. Cold Spring Harb Symp Quant Biol. 1968;33:809–822. doi: 10.1101/sqb.1968.033.01.093. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Grindley N. D., Anderson E. S. DNA--protein complexes of delta-mediated transfer systems. Biochim Biophys Acta. 1972 Dec 6;287(2):355–360. doi: 10.1016/0005-2787(72)90385-1. [DOI] [PubMed] [Google Scholar]
- KJELDGAARD N. O., MAALOE O., SCHAECHTER M. The transition between different physiological states during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):607–616. doi: 10.1099/00221287-19-3-607. [DOI] [PubMed] [Google Scholar]
- Kool A. J., van Zeben M. S., Nijkamp H. J. Identification of messenger ribonucleic acids and proteins synthesized by the bacteriocinogenic factor Clo DF13 in purified minicells of Escherichia coli. J Bacteriol. 1974 Apr;118(1):213–224. doi: 10.1128/jb.118.1.213-224.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Punch J. D. The problems of drug-resistant pathogenic bacteria. Regulation of R-factor replication in Proteus mirabilis. Ann N Y Acad Sci. 1971 Jun 11;182:201–216. doi: 10.1111/j.1749-6632.1971.tb30657.x. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E., Freedman M. L. Chromosome replication and the division cycle of Escherichia coli B-r. J Bacteriol. 1971 Jul;107(1):95–99. doi: 10.1128/jb.107.1.95-99.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch-Portnoy Y. M., Miklos G. L., Helinski D. R. Properties of the relaxation complexes of supercoiled deoxyribonucleic acid and protein of the R plasmids R64, R28K, and R6K. J Bacteriol. 1974 Oct;120(1):545–548. doi: 10.1128/jb.120.1.545-548.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lark C. Regulation of deoxyribonucleic acid synthesis in Escherichia coli: dependence on growth rates. Biochim Biophys Acta. 1966 Jun 22;119(3):517–525. doi: 10.1016/0005-2787(66)90128-6. [DOI] [PubMed] [Google Scholar]
- Lindström E. B., Nordström K. Automated method for determination of penicillins, cephalosporins, and penicillinases. Antimicrob Agents Chemother. 1972 Feb;1(2):100–106. doi: 10.1128/aac.1.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Morris C. F., Hashimoto H., Mickel S., Rownd R. Round of replication mutant of a drug resistance factor. J Bacteriol. 1974 Jun;118(3):855–866. doi: 10.1128/jb.118.3.855-866.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Ingram L. C., Lundbäck A. Mutations in R factors of Escherichia coli causing an increased number of R-factor copies per chromosome. J Bacteriol. 1972 May;110(2):562–569. doi: 10.1128/jb.110.2.562-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström U. M., Engberg B., Nordström K. Competition for DNA polymerase III between the chromosome and the R-factor R1. Mol Gen Genet. 1974;135(3):185–190. doi: 10.1007/BF00268614. [DOI] [PubMed] [Google Scholar]
- Pritchard R. H. Review lecture on the growth and form of a bacterial cell. Philos Trans R Soc Lond B Biol Sci. 1974 Feb 21;267(886):303–336. doi: 10.1098/rstb.1974.0003. [DOI] [PubMed] [Google Scholar]
- Rosenberg B. H., Cavalieri L. F., Ungers G. The negative control mechanism for E. coli DNA replication. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1410–1417. doi: 10.1073/pnas.63.4.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R. Replication of a bacterial episome under relaxed control. J Mol Biol. 1969 Sep 28;44(3):387–402. doi: 10.1016/0022-2836(69)90368-4. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Falkow S. Specific labeling and physical characterization of R-factor deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):331–339. doi: 10.1128/jb.104.1.331-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L., Maaloe O. Autorepressor model for control of DNA replication. Nat New Biol. 1973 Jan 31;241(109):133–135. doi: 10.1038/newbio241133a0. [DOI] [PubMed] [Google Scholar]
- Thompson R., Broda P. DNA polymerase 3 and the replication of F and ColVBtrp in Escherichia coli K-12. Mol Gen Genet. 1973 Dec 31;127(3):255–258. doi: 10.1007/BF00333765. [DOI] [PubMed] [Google Scholar]
- Timmis K., Winkler U. Gene dosage studies with pleiotropic mutants of Serratia marcescens superactive in the synthesis of marcescin A and certain other exocellular proteins. Mol Gen Genet. 1973 Aug 17;124(3):207–217. doi: 10.1007/BF00293092. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Zeuthen J., Pato M. L. Replication of the F'lac sex factor in the cell cycle of Escherichia coli. Mol Gen Genet. 1971;111(3):242–255. doi: 10.1007/BF00433109. [DOI] [PubMed] [Google Scholar]



