Abstract
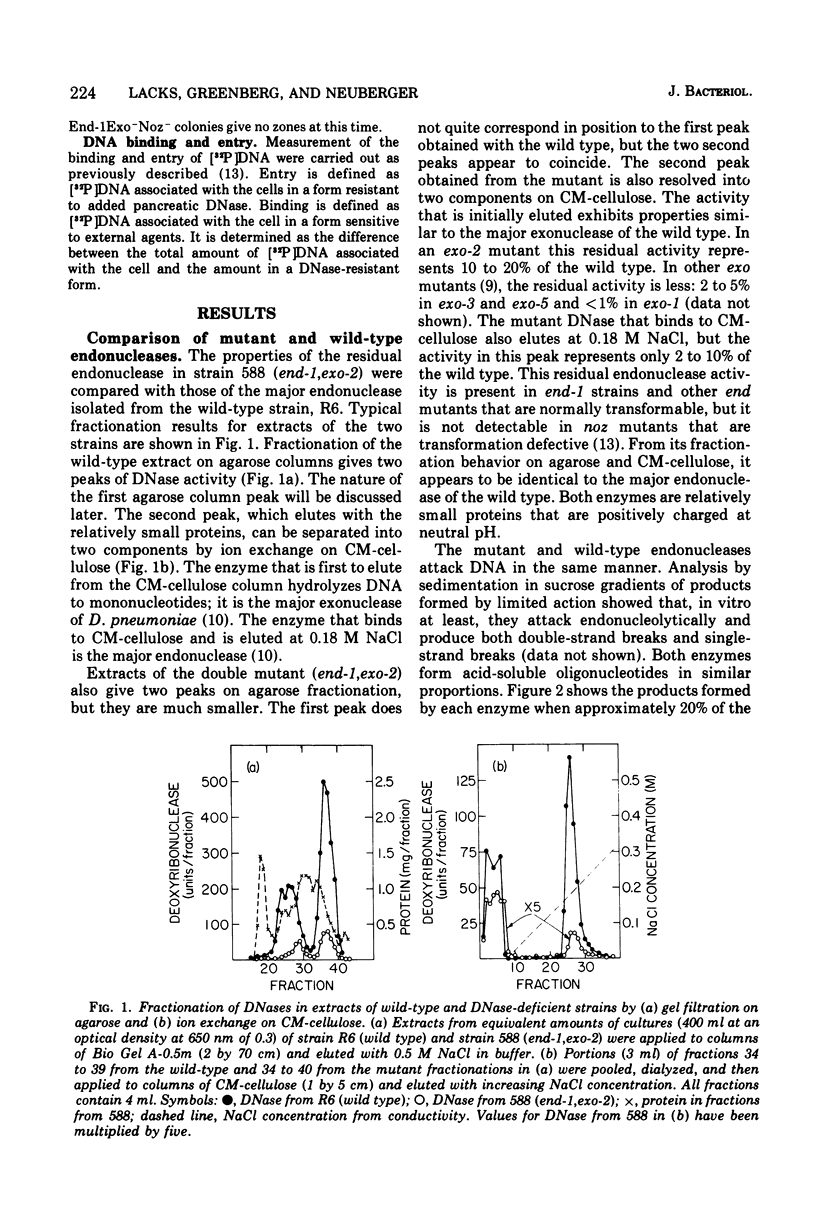
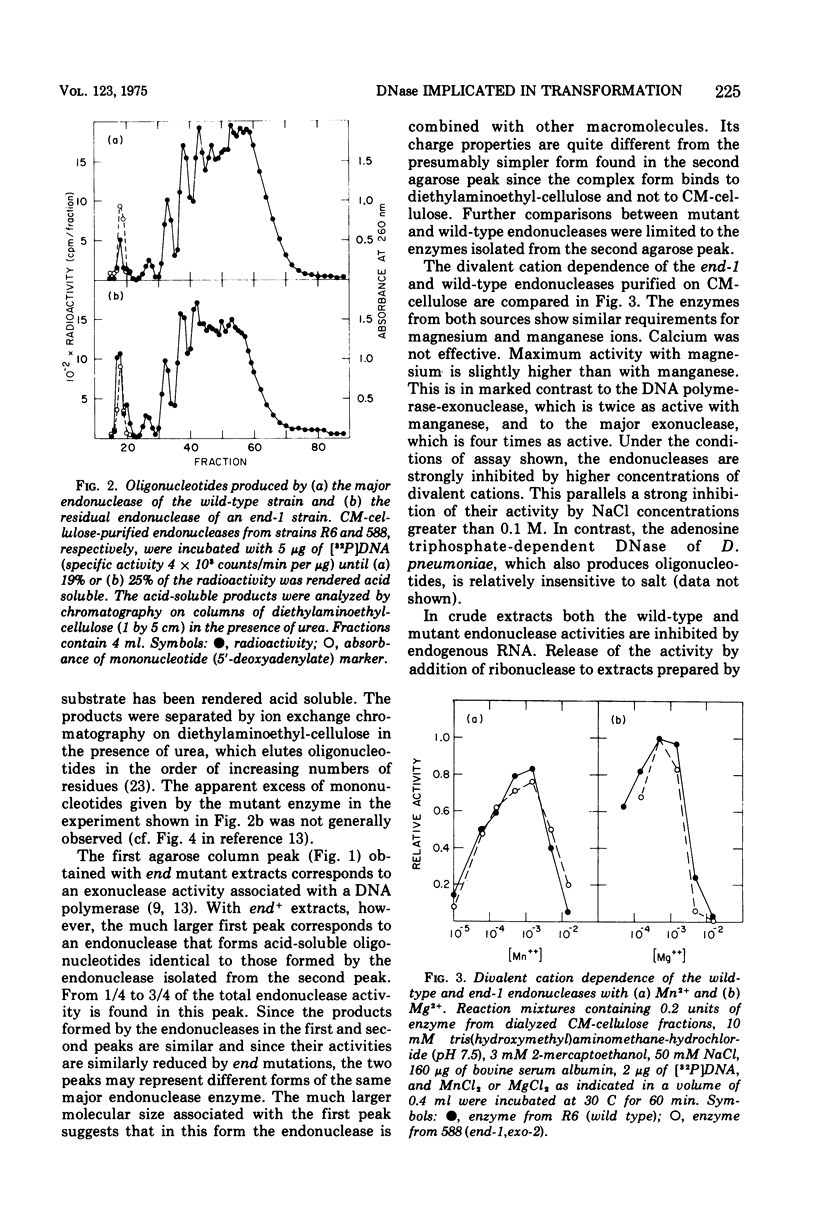
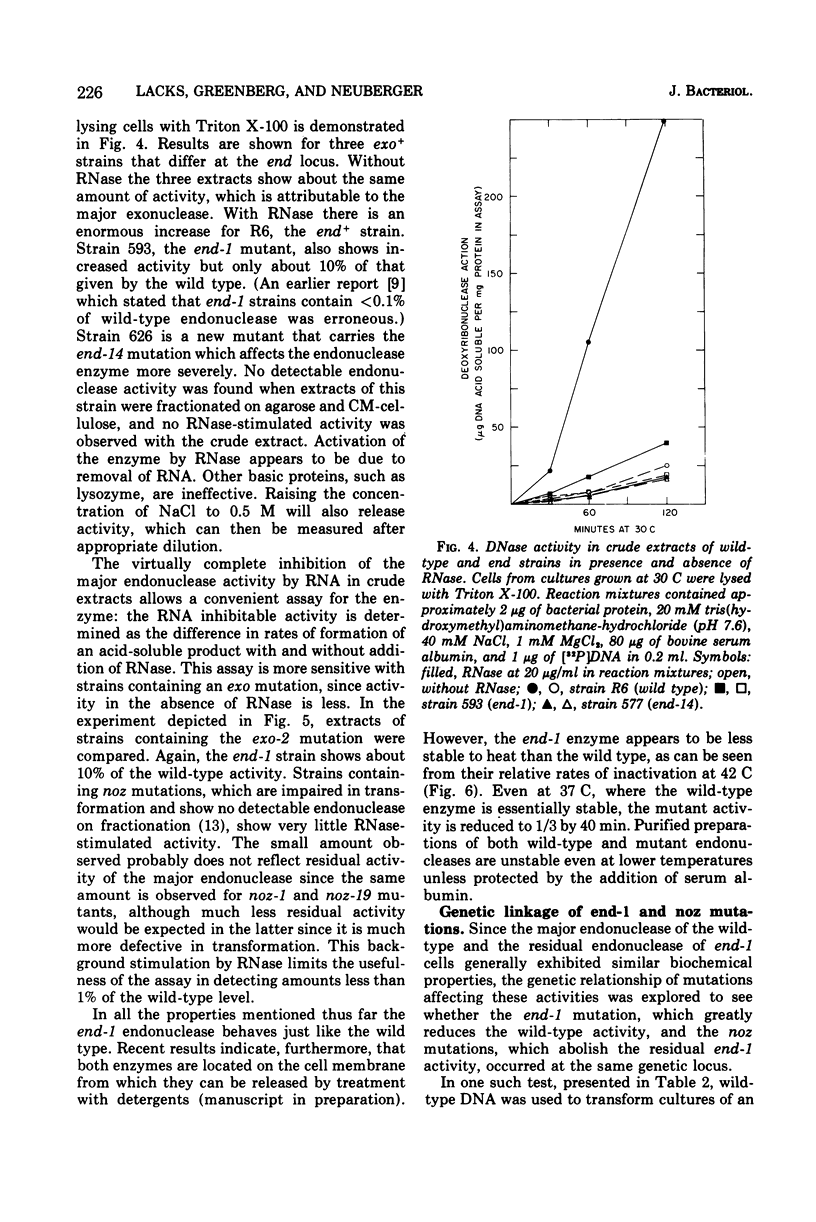
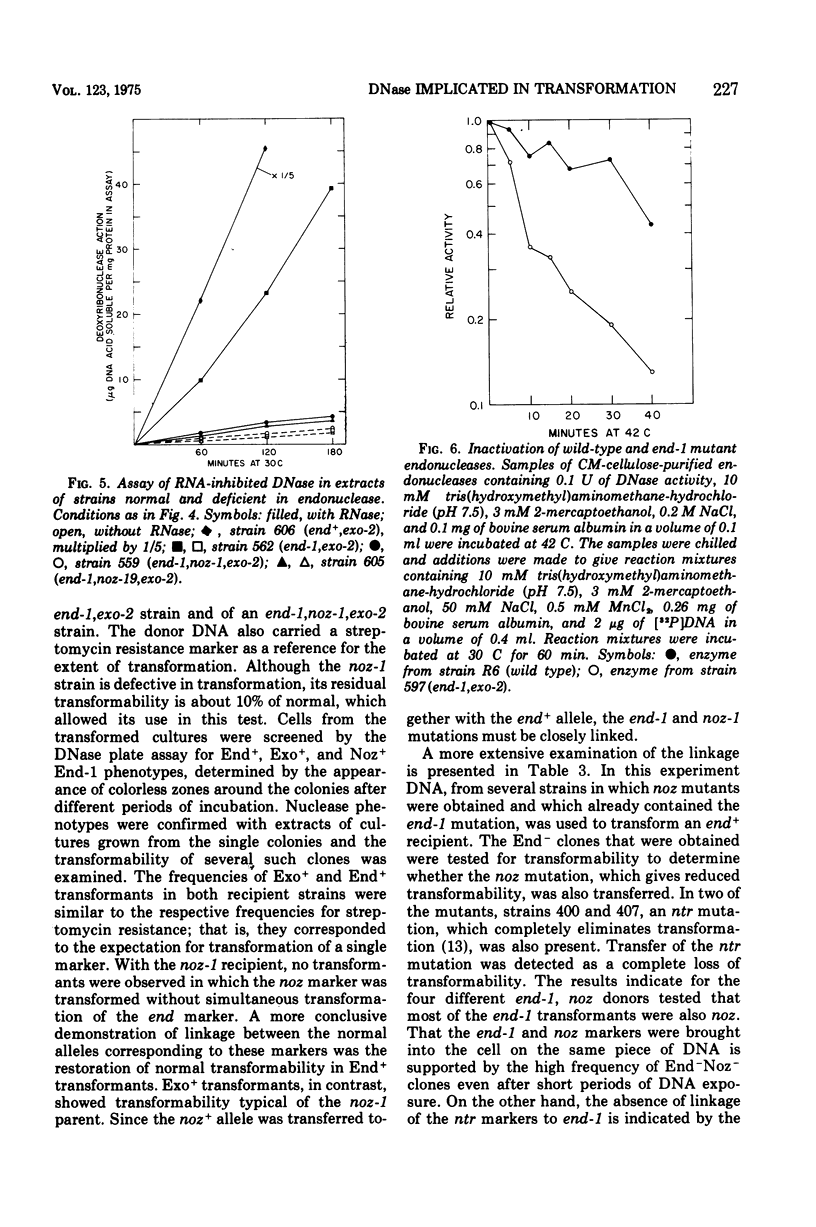
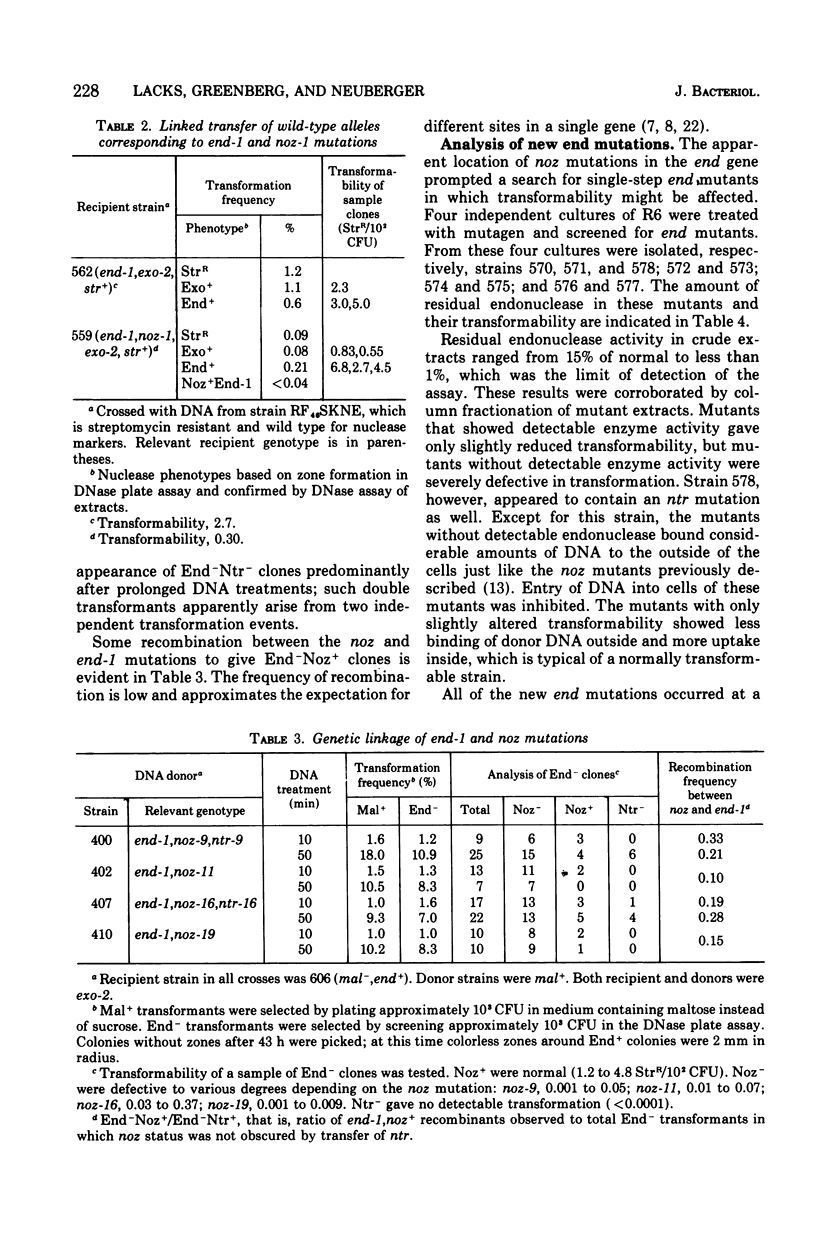
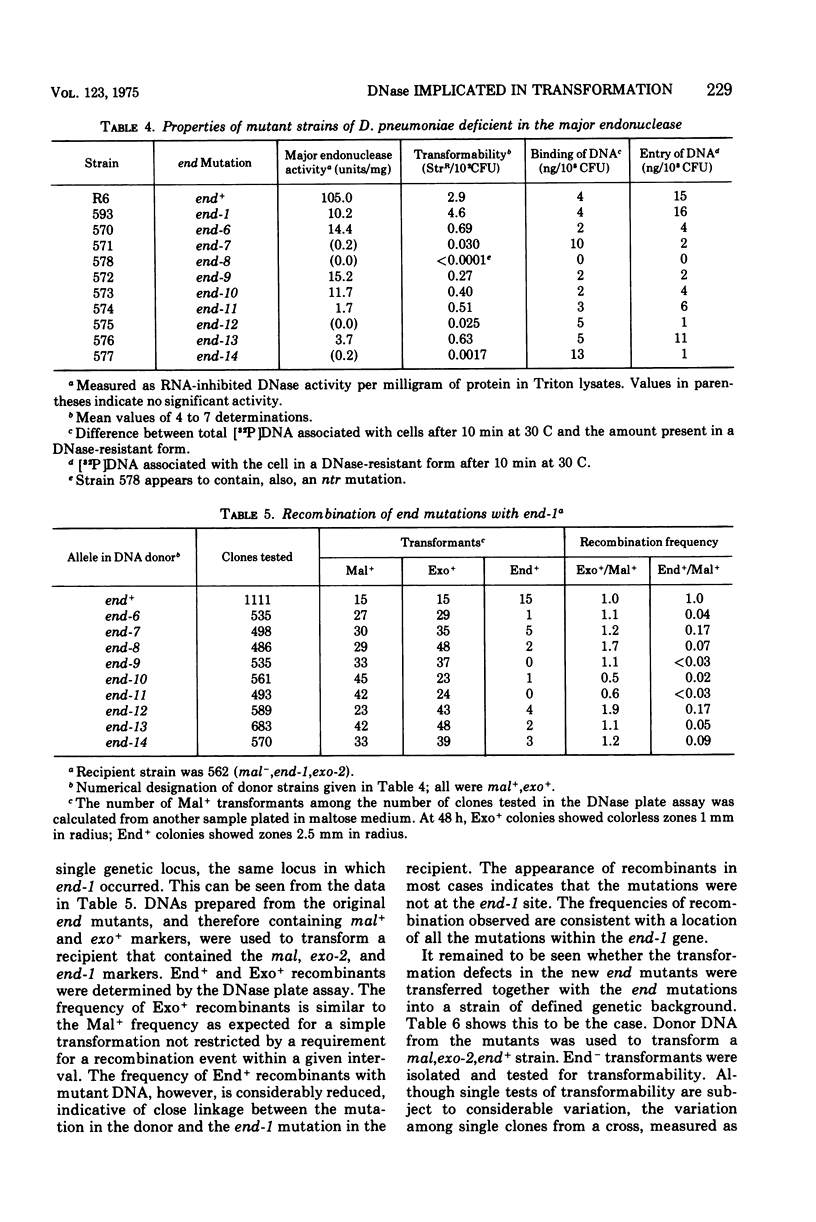
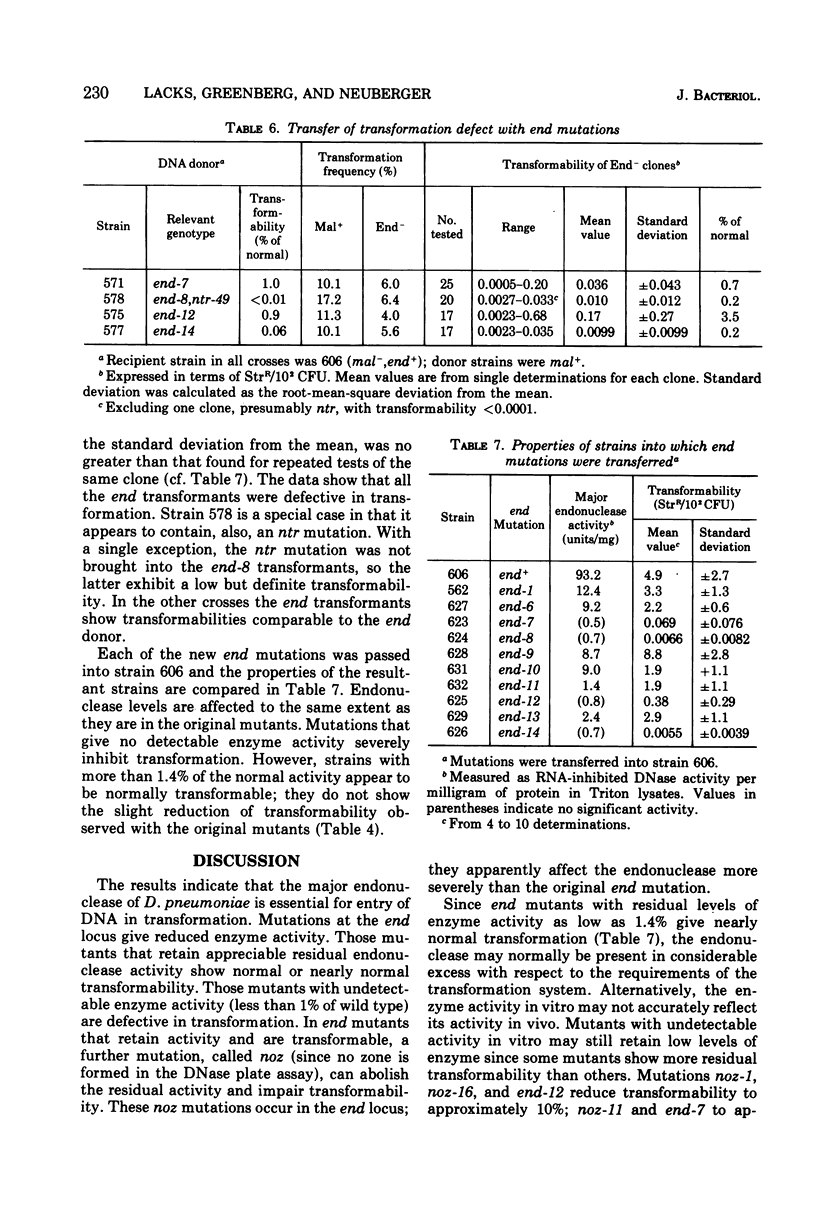
A mutation of Diplococcus pneumoniae, end-1, reduces the major deoxyribonuclease activity of the cell, an endonuclease, to 10% of its normal value without impairing transformation. Further mutations, called noz, abolish the residual endonuclease activity and block transformation. The residual endonuclease is similar to the wild-type enzyme in size, charge, divalent cation dependence, inhibition by ribonucleic acid, and formation of oligonucleotide products. However, the mutant endonuclease is more temperature sensitive, which suggests that the end-1 mutation occurred in a structural gene for the enzyme. Genetic analysis showed that the noz mutations occur at the same genetic locus. A number of new end mutants were analyzed. Those that retained more than 1.4% of the normal endonuclease activity were essentially normal in transformation; those with less than 1% were defective. The transformation-defective end mutants appear to be blocked in the entry of deoxyribonucleic acid (DNA) since they carry out the prior step of binding DNA to the outside of the cell. The major endonuclease of the cell may act as a DNA translocase by attacking and degrading one strand of DNA, thereby facilitating entry of the complementary strand into the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hotchkiss R. D. CYCLICAL BEHAVIOR IN PNEUMOCOCCAL GROWTH AND TRANSFORMABILITY OCCASIONED BY ENVIRONMENTAL CHANGES. Proc Natl Acad Sci U S A. 1954 Feb;40(2):49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohoutová M. Role of deoxyribonuclease in genetic transformation. I. Deoxyribonuclease activity in variously transformable strains. Folia Microbiol (Praha) 1967;12(4):311–315. doi: 10.1007/BF02873805. [DOI] [PubMed] [Google Scholar]
- Kohoutová M. Role of deoxyribonuclease in genetic transformation. II. RNA as an inhibitor of doexyribonuclease activity in pneumococcal strains. Folia Microbiol (Praha) 1967;12(4):316–322. doi: 10.1007/BF02873806. [DOI] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., ROUSSOS G. G., PRATT E. A. The deoxyribonucleases of Escherichia coli. II. Purification and properties of a ribonucleic acid-inhibitable endonuclease. J Biol Chem. 1962 Mar;237:819–828. [PubMed] [Google Scholar]
- Lacks S. Genetic regulation of maltosaccharide utilization in Pneumococcus. Genetics. 1968 Dec;60(4):685–706. doi: 10.1093/genetics/60.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Competence for deoxyribonucleic acid uptake and deoxyribonuclease action external to cells in the genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1973 Apr;114(1):152–163. doi: 10.1128/jb.114.1.152-163.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Deoxyribonucleases of Pneumococcus. J Biol Chem. 1967 Jul 10;242(13):3108–3120. [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Role of a deoxyribonuclease in the genetic transformation of Diplococcus pneumoniae. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2305–2309. doi: 10.1073/pnas.71.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Breakage prior to entry of donor DNA in Pneumococcus transformation. Biochim Biophys Acta. 1973 Apr 11;299(4):545–556. doi: 10.1016/0005-2787(73)90226-8. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Transformation and deoxyribonucleic acid size: extent of degradation on entry varies with size of donor. J Bacteriol. 1972 Dec;112(3):1157–1168. doi: 10.1128/jb.112.3.1157-1168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalecz J., Dobrzański W. T. Correlation between the occurrence of competence in the transformation of group H Streptococci and the presence of the competence factor and the in vitro DNA-inactivating factor. Mol Gen Genet. 1972;114(3):249–260. doi: 10.1007/BF01788894. [DOI] [PubMed] [Google Scholar]
- OTTOLENGHI E., HOTCHKISS R. D. Release of genetic transforming agent from pneumococcal cultures during growth and disintegration. J Exp Med. 1962 Oct 1;116:491–519. doi: 10.1084/jem.116.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M., Cosloy S. D. The genetic and biochemical basis of the transformability of Escherichia coli K12. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1568–1572. doi: 10.1016/0006-291x(72)90520-7. [DOI] [PubMed] [Google Scholar]
- Pakula R., Spencer L. R., Goldstein P. A. Deoxyribonuclease and competence factor activities of transformable and nontransformable group H streptococci. Can J Microbiol. 1972 Feb;18(2):111–119. doi: 10.1139/m72-020. [DOI] [PubMed] [Google Scholar]
- Seto H., Tomasz A. Early stages in DNA binding and uptake during genetic transformation of pneumococci. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1493–1498. doi: 10.1073/pnas.71.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M., Hachtel S. L. Increased dihydrofolate reductase synthess in Diplococcus pneumoniae following translatable alteration of the structural gene. I. Genotype derivation and recombinational analyses. Genetics. 1969 Feb;61(2):293–312. doi: 10.1093/genetics/61.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vovis G. F. Adenosine triphosphate-dependent deoxyribonuclease from Diplococcus pneumoniae: fate of transforming deoxyribonucleic acid in a strain deficient in the enzymatic activity. J Bacteriol. 1973 Feb;113(2):718–723. doi: 10.1128/jb.113.2.718-723.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


