Abstract
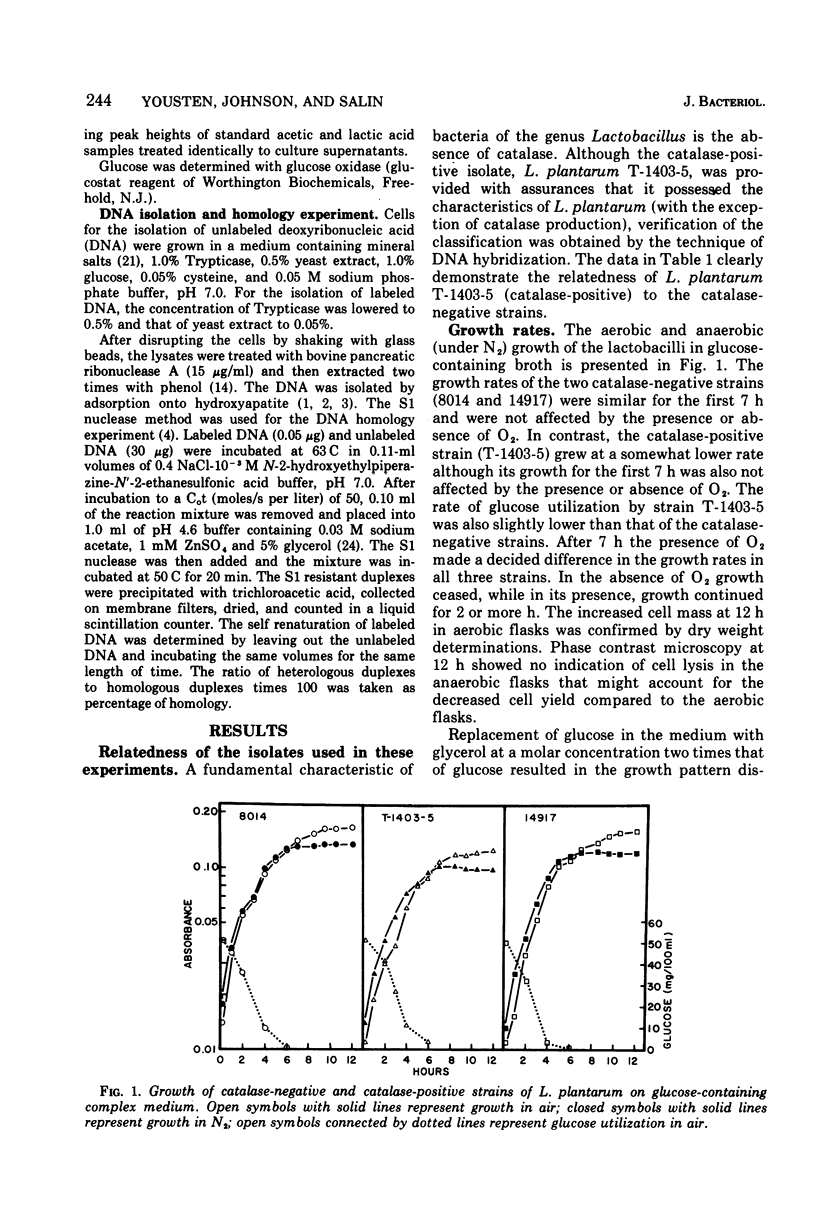
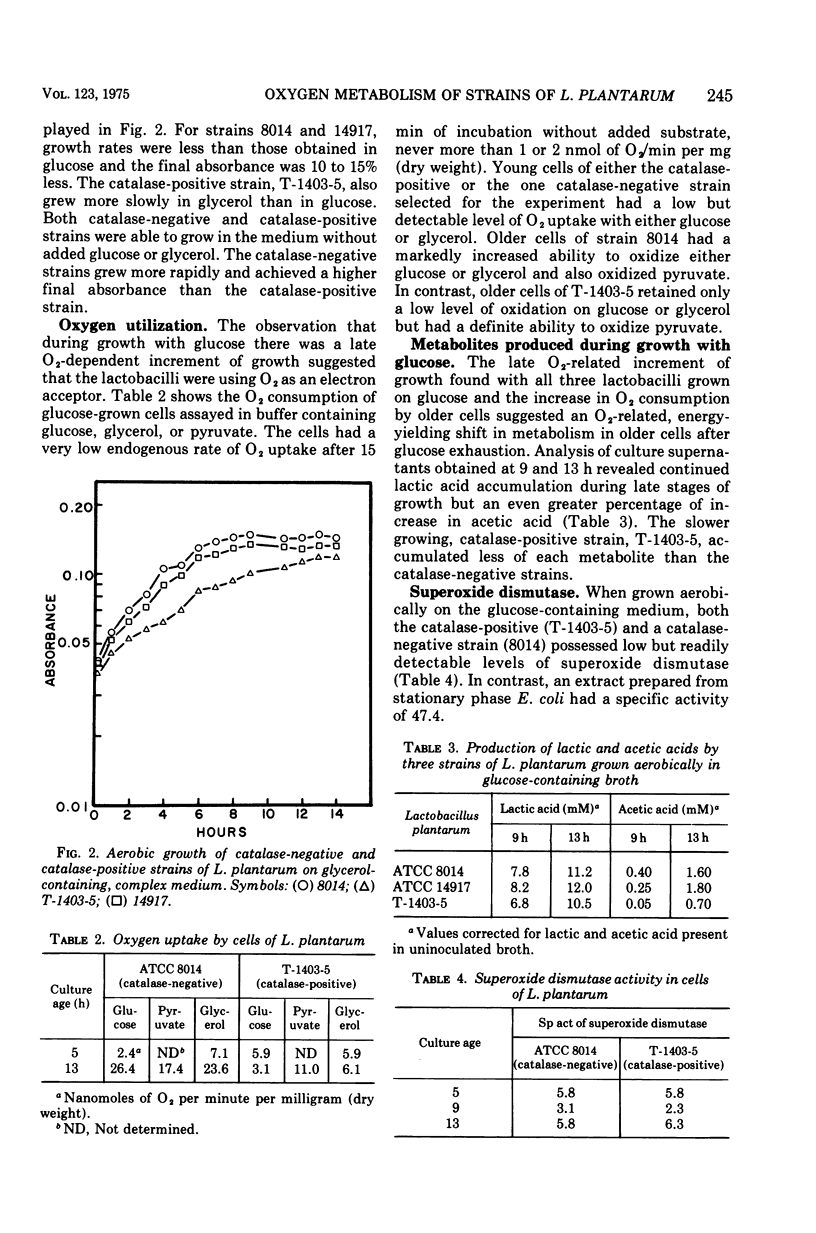
Two catalase-negative strains of Lactobacillus plantarum and a strain producing the atypical, nonheme catalase were studied to determine if the ability to produce the atypical catalase conferred any growth advantage upon the producing strain. Both catalase-negative strains grew more rapidly than the catalase-positive strain under aerobic or anaerobic conditions in a glucose-containing, complex medium. Upon exhaustion of glucose from the medium, all three strains continued growth under aerobic but not under anaerobic conditions. The continued aerobic growth was accompanied by production of acetic acid in addition to the lactic acid produced during growth on glucose. Oxygen was taken up by exponential phase-cell suspensions grown on glucose when glucose or glycerol were used as substrates. Cells harvested from glucose-exhausted medium oxidized glucose, glycerol, and pyruvate. Oxygen utilization by a catalase-negative strain increased as did the specific activity of reduced nicotinamide adenine dinucleotide peroxidase during late growth in the glucose-exhausted medium. The catalase-positive strain and the catalase-negative strain tested both possessed low but readily detectable levels of superoxide dismutase throughout growth. The growth responses are discussed in terms of the presence of enzymes which would allow the cells to remove potentially damaging reduction products of O2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi G. Chromatography of nucleic acids on hydroxyapatite. 3. Chromatography of RNA and polyribonucleotides. Biochim Biophys Acta. 1969 Feb 18;174(2):449–457. doi: 10.1016/0005-2787(69)90275-5. [DOI] [PubMed] [Google Scholar]
- Bernardi G. Chromatography of nucleic acids on hydroxyapatite. I. Chromatography of native DNA. Biochim Biophys Acta. 1969 Feb 18;174(2):423–434. doi: 10.1016/0005-2787(69)90273-1. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Rake A. V., Johnson K. E. Batch procedure for thermal elution of DNA from hydroxyapatite. Anal Biochem. 1969 Apr 4;28(1):447–459. doi: 10.1016/0003-2697(69)90199-7. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Brenner D. J., Falkow S. Use of a single-strand specific nuclease for analysis of bacterial and plasmid deoxyribonucleic acid homo- and heteroduplexes. J Bacteriol. 1973 Sep;115(3):904–911. doi: 10.1128/jb.115.3.904-911.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBROGOSZ W. J., STONE R. W. Oxidative metabolism in Pediococcus pentosaceus. I. Role of oxygen and catalase. J Bacteriol. 1962 Oct;84:716–723. doi: 10.1128/jb.84.4.716-723.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirar H., Collins E. B. Aerobic utilization of low concentrations of galactose by Lactobacillus plantarum. J Gen Microbiol. 1973 Oct;78(2):211–215. doi: 10.1099/00221287-78-2-211. [DOI] [PubMed] [Google Scholar]
- Dirar H., Collins E. B. End-products, fermentation balances and molar growth yields of homofermentative lactobacilli. J Gen Microbiol. 1972 Nov;73(2):233–238. doi: 10.1099/00221287-73-2-233. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen metabolism in Lactobacillus plantarum. J Bacteriol. 1974 Jan;117(1):166–169. doi: 10.1128/jb.117.1.166-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Fridovich I. Superoxide dismutases of Escherichia coli: intracellular localization and functions. J Bacteriol. 1973 Sep;115(3):987–991. doi: 10.1128/jb.115.3.987-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus I. C., Sherman J. M. The Fermentation of Glycerol by Streptococci. J Bacteriol. 1943 Feb;45(2):155–162. doi: 10.1128/jb.45.2.155-162.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. DISTRIBUTION AND CHARACTERISTICS OF THE CATALASES OF LACTOBACILLACEAE. J Bacteriol. 1965 Aug;90:347–351. doi: 10.1128/jb.90.2.347-351.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. ISOLATION AND CHARACTERIZATION OF THE CYANIDE-RESISTANT AND AZIDE-RESISTANT CATALASE OF LACTOBACILLUS PLANTARUM. J Bacteriol. 1965 Aug;90:352–356. doi: 10.1128/jb.90.2.352-356.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Cummins C. S. Cell wall composition and deoxyribonucleic acid similarities among the anaerobic coryneforms, classical propionibacteria, and strains of Arachnia propionica. J Bacteriol. 1972 Mar;109(3):1047–1066. doi: 10.1128/jb.109.3.1047-1066.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Aerobic metabolism of Streptococcus agalactiae. J Bacteriol. 1967 Jul;94(1):184–191. doi: 10.1128/jb.94.1.184-191.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley A. J., Jahrling P., Van Demark P. J. Molar growth yields as evidence for oxidative phosphorylation in Streptococcus faecalis strain 10Cl. J Bacteriol. 1968 Nov;96(5):1595–1600. doi: 10.1128/jb.96.5.1595-1600.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- WHITTENBURY R. HYDROGEN PEROXIDE FORMATION AND CATALASE ACTIVITY IN THE LACTIC ACID BACTERIA. J Gen Microbiol. 1964 Apr;35:13–26. doi: 10.1099/00221287-35-1-13. [DOI] [PubMed] [Google Scholar]
- Walker G. A., Kilgour G. L. Pyridine nucleotide oxidizing enzymes of Lactobacillus casei. II. Oxidase and peroxidase. Arch Biochem Biophys. 1965 Sep;111(3):534–539. doi: 10.1016/0003-9861(65)90232-8. [DOI] [PubMed] [Google Scholar]
- Yousten A. A., Bulla L. A., Jr, McCord J. M. Superoxide dismutase in Bacillus popilliae, a catalaseless aerobe. J Bacteriol. 1973 Jan;113(1):524–525. doi: 10.1128/jb.113.1.524-525.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


