Abstract
Respiratory nitrate reductase with lactate as a hydrogen donor has been studied in cells and spheroplast preparations of wild type and heme-deficienct mutants of Staphylococcus aureus. The activity is rapidly induced when suspensions of aerobically grown cells are incubated without aeration in a complete medium with nitrate. In ruptured spheroplast preparations, the activity with lactate as the donor is located in the membrane fraction, whereas at least 50% of the activity assayed with reduced benzyl viologen is in the cytoplasm. The reductase is inhibited by azide and cyanide, and the lactate-linked system is also sensitive to oxamate, 2-heptyl-4-hydroxyquinoline-N-oxide, dicoumarol, and p-chloromercuribenzoate. An inactive form of the reductase is apparently made during induction with tungstate; this can be activated by subsequent incubation with molybdate in the presence of chloramphenicol. Nitrate reductase activity with reduced benzyl viologen as the donor is induced in suspensions of heme-deficient mutants in the presence or absence of heme. The proportion of cytoplasmic activity is increased in the absence of heme. The staphylococcal nitrate reductase has many of the characteristics commonly associated with the respiratory enzyme in other organisms, but the apparent predominance of cytoplasmic activity is unusual.
Full text
PDF

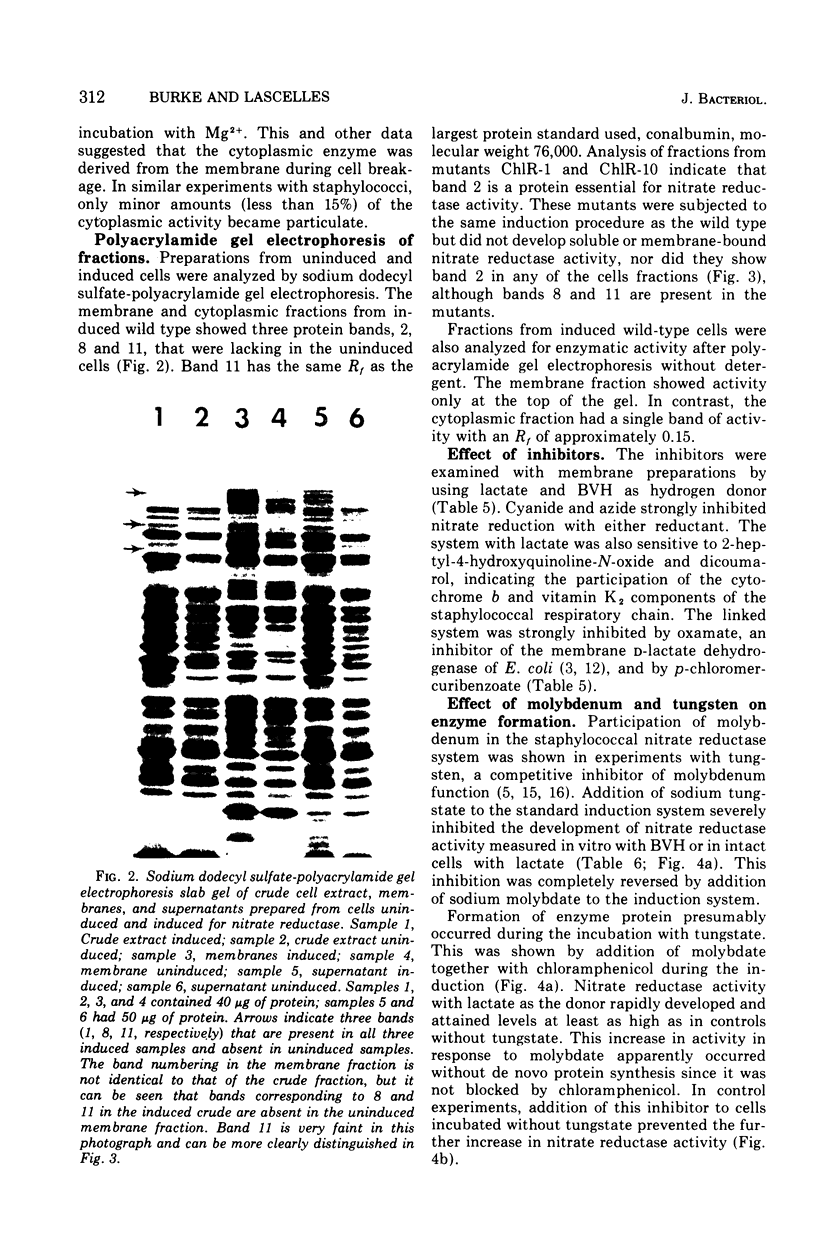
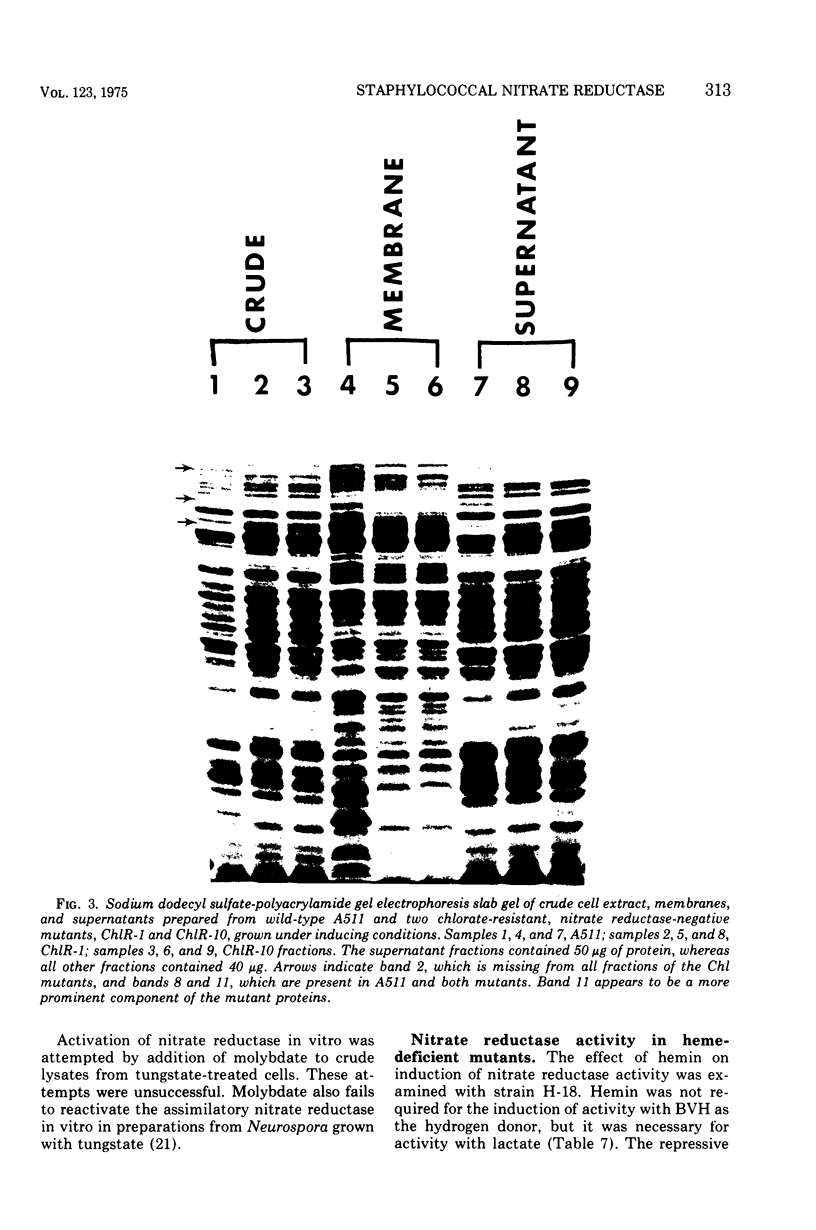
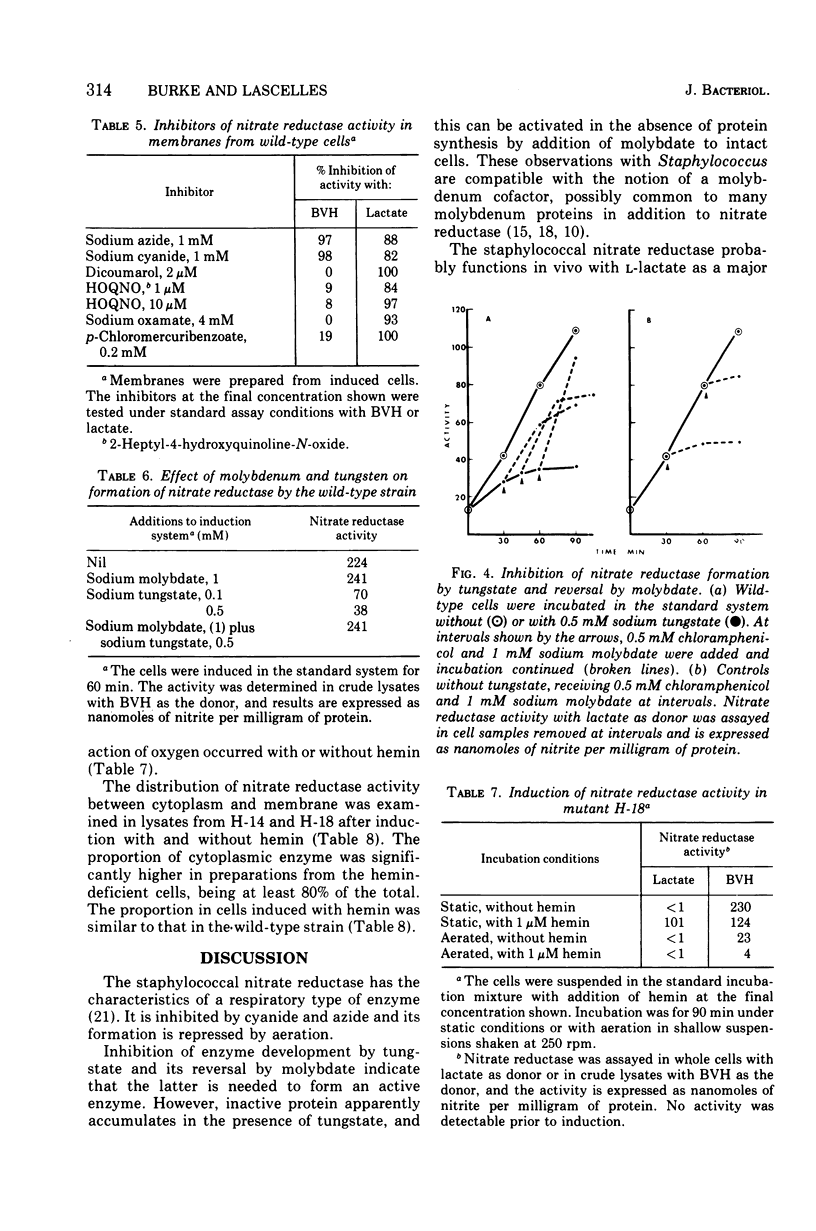

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Azoulay E., Puig J., Pichinoty F. Alteration of respiratory particles by mutation in Escherichia coli K 12. Biochem Biophys Res Commun. 1967 Apr 20;27(2):270–274. doi: 10.1016/s0006-291x(67)80073-1. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. I. The site of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in Escherichia coli membrane vesicles. J Biol Chem. 1971 Sep 10;246(17):5518–5522. [PubMed] [Google Scholar]
- CHANG J. P., LASCELLES J. NITRATE REDUCTASE IN CELL-FREE EXTRACTS OF A HAEMIN-REQUIRING STRAIN OF STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Dec;89:503–510. doi: 10.1042/bj0890503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. Effects of molybdate, tungstate, and selenium compounds on formate dehydrogenase and other enzyme systems in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1032–1040. doi: 10.1128/jb.110.3.1032-1040.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDNER J. F., LASCELLES J. The requirement for acetate of a streptomycin-resistant strain of Staphylococcus aureus. J Gen Microbiol. 1962 Sep;29:157–164. doi: 10.1099/00221287-29-1-157. [DOI] [PubMed] [Google Scholar]
- Garrard W., Lascelles J. Regulation of Staphylococcus aureus lactate dehydrogenase. J Bacteriol. 1968 Jan;95(1):152–156. doi: 10.1128/jb.95.1.152-156.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier D. K., Clark-Walker G. D., Garrard W. T., Jr, Lascelles J. Nitrate reductase and soluble cytochrome c in Spirillum itersonii. J Bacteriol. 1970 Jun;102(3):790–801. doi: 10.1128/jb.102.3.797-803.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenbaum P. E., White D. C. Role of lipid in the formation and function of the respiratory system of Staphylococcus aureus. Ann N Y Acad Sci. 1974 Jul 31;236(0):115–123. doi: 10.1111/j.1749-6632.1974.tb41486.x. [DOI] [PubMed] [Google Scholar]
- HAGER L. P., KORNBERG H. L. On the mechanism of alpha-oxoglutarate oxidation in Escherichia coli. Biochem J. 1961 Jan;78:194–198. doi: 10.1042/bj0780194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Schairer H. U. Electron-transport chains of Escherichia coli. Reconstitution of respiration in a 5-aminolaevulinic acid-requiring mutant. Eur J Biochem. 1973 May;35(1):34–45. doi: 10.1111/j.1432-1033.1973.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. XV. Purification and properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1973 Oct 25;248(20):7012–7017. [PubMed] [Google Scholar]
- Konings W. N., Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. 2. The coupling of reduced phenazine methosulfate to the concentrative uptake of beta-galactosides and amino acids. J Biol Chem. 1971 Oct 10;246(19):5857–5861. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lascelles J. Anaerobic growth requirements of staphylococci and the enzymes of pyrimidine synthesis. Ann N Y Acad Sci. 1974 Jul 31;236(0):96–104. doi: 10.1111/j.1749-6632.1974.tb41484.x. [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Erickson R., Pan S. S., Jones G., May F., Nason A. Effect of tungsten and vanadium on the in vitro assembly of assimilatory nitrate reductase utilizing Neurospora mutant nit-1. J Biol Chem. 1974 Jun 25;249(12):3953–3959. [PubMed] [Google Scholar]
- Lee K. Y., Pan S. S., Erickson R., Nason A. Involvement of molybdenum and iron in the in vitro assembly of assimilatory nitrate reductase utilizing Neurospora mutant nit-1. J Biol Chem. 1974 Jun 25;249(12):3941–3952. [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A. Reconstitution of nitrate reductase activity and formation of membrane particles from cytoplasmic extracts of chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1164–1176. doi: 10.1128/jb.114.3.1164-1176.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A. Restoration of reduced nicotinamide adenine dinucleotide phosphate-nitrate reductase activity of a Neurospora mutant by extracts of various chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1972 Oct;112(1):388–391. doi: 10.1128/jb.112.1.388-391.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Fitzgerald J. W., Dodgson K. S. Methods for visualization of enzymes in polyacrylamide gels. Appl Microbiol. 1974 Jan;27(1):154–158. doi: 10.1128/am.27.1.154-158.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Showe M. K., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: isolation and characterization of mutants unable to reduce nitrate. J Bacteriol. 1969 Mar;97(3):1291–1297. doi: 10.1128/jb.97.3.1291-1297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasarman A., Purvis P., Portelance V. Role of menaquinone in nitrate respiration in Staphylococcus aureus. J Bacteriol. 1974 Feb;117(2):911–913. doi: 10.1128/jb.117.2.911-913.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. Further studies on amino acid transport in Staphylococcus aureus membrane vesicles. J Biol Chem. 1974 Jul 10;249(13):4275–4281. [PubMed] [Google Scholar]
- Tien W., White D. C. Linear sequential arrangement of genes for the biosynthetic pathway of protoheme in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1392–1398. doi: 10.1073/pnas.61.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]