Abstract
The plant hormone indoleacetic acid (IAA) transcriptionally activates early genes in plants. The Aux/IAA family of early genes encodes proteins that are short-lived and nuclear-localized. They also contain a putative prokaryotic βαα DNA binding motif whose formation requires protein dimerization. Here, we show that the pea PS-IAA4 and Arabidopsis IAA1 and IAA2 proteins perform homo- and heterotypic interactions in yeast using the two-hybrid system. Gel-filtration chromatography and chemical cross-linking experiments demonstrate that the PS-IAA4 and IAA1 proteins interact to form homodimers in vitro. Deletion analysis of PS-IAA4 indicates that the βαα containing acidic C terminus of the protein is necessary for homotypic interactions in the yeast two-hybrid system. Screening an Arabidopsis λ-ACT cDNA library using IAA1 as a bait reveals heterotypic interactions of IAA1 with known and newly discovered members of the Arabidopsis Aux/IAA gene family. The new member IAA24 has similarity to ARF1, a transcription factor that binds to an auxin response element. Combinatorial interactions among the various members of the Aux/IAA gene family may regulate a variety of late genes as well as serve as autoregulators of early auxin-regulated gene expression. These interactions provide a molecular basis for the developmental and tissue-specific manner of auxin action.
Keywords: auxin signaling, yeast two-hybrid system, protein cross-linking, β-sheet DNA binding motif, combinatorial interactions
The plant growth hormone auxin typified by indoleacetic acid (IAA) regulates various aspects of plant growth and development (1, 2). Auxin transcriptionally activates early genes that are thought to be responsible for mediating the various auxin effects (3–5). Four different families of early genes have been identified in various plant species during the last ten years (for review, see ref. 6). The members of the first family encode glutathione S-transferases. The second family, represented by GH3, may encode proteins that affect auxin homeostasis (G. Hagen and T. Guilfoyle, personal communication). The SAURs genes are members of the third family, and the function of their encoded polypeptides is unknown. Finally, the Aux/IAA gene family, represented by PS-IAA4, the most thoroughly studied family member, encodes short-lived nuclear proteins. These proteins contain a putative βαα DNA recognition motif found in the prokaryotic repressors of the Arc family (7). Based on this motif, it has been proposed that the Aux/IAA gene products may be transcriptional regulators of late genes responsible for mediating the various auxin responses (7). Structural analysis of the Arc and MetJ repressors has shown that the antiparallel β-sheet responsible for DNA binding is formed by protein homodimerization and that the second helix of the motif is responsible for this interaction (8). There is no evidence to indicate either that the Aux/IAA gene products form homodimers or that they bind DNA. Here we show that the Aux/IAA proteins have the ability to form homodimers in vivo using the yeast two-hybrid system (9) and in vitro using biochemical experiments. Furthermore, a two-hybrid screen with IAA1 as bait and a Gal4 transactivation domain-tagged Arabidopsis cDNA library reveals that heterotypic interaction occurs among highly divergent Aux/IAA gene products.
MATERIALS AND METHODS
Bacterial, Yeast Strains, and Plant Material.
Escherichia coli DH5α was used for plasmid propagation. E. coli MH4 was used to recover the Gal4 activation domain (Gal4AD) plasmid from yeast (10). Yeast Y190 was used as the host strain for the yeast two-hybrid system (11). Arabidopsis thaliana (ecotype Columbia) was germinated and grown in liquid culture as described (12).
Plasmid Construction.
Full-length cDNAs of IAA1 and IAA2 (Arabidopsis) and PS-IAA4 (pea) were generated by PCR and subcloned in frame into the yeast vectors pGBT9.BS and pGAD.GH giving rise to pGal4BD-IAA1 (-IAA2 and -PS-IAA4) and pGal4AD-IAA1 (-IAA2 and -PS-IAA4), respectively. The following deletions of PS-IAA4 were made by PCR and fused in frame into the Gal4 DNA binding domain (Gal4BD) of pGBT9.BS and into Gal4AD of pGAD.GH; deletion 2 (aa 1–85); deletion 3 (aa 1–137); deletion 4 (aa 86–137); deletion 5 (aa 138–189); and deletion 6 (aa 86–189). All subcloned PCR products were verified by sequencing. The plasmids of Gal4BD-SNF4 in pAS and Gal4AD-SNF1 in pACT were the gifts from S. Elledge (13).
Quantitation of β-Galactosidase (β-gal) Activity.
β-Gal activity was assayed using chlorophenyl-red-β-d-galactopyranoside (Boehringer Mannheim) as substrate as described (14, 15). β-Gal activity (units) was calculated as follows: OD574 of the supernatants × 1,000/reaction time (min) × culture volume used for the assay (ml) × OD600 of the culture.
Construction of Arabidopsis cDNA Library in λ-ACT.
The construction of cDNA library was done essentially as described (13, 16). Poly(A) RNA was isolated from 3-day-old etiolated Arabidopsis. First-stranded cDNA was synthesized at 37°C from 5 μg of poly(A) RNA with oligo(dT) and 1,000 units of Superscript (BRL). After the second-strand reaction, the cDNA was precipitated with spermine and washed with spermine wash buffer. The cDNA was dissolved in 40 μl of TE buffer and made flush with T4 DNA polymerase according to the supplier’s instructions (New England Biolabs). After inactivation of the enzyme by the addition of 5 μl of 0.5 M EDTA, samples were extracted with phenol/chloroform and precipitated with ethanol. The cDNA was resuspended in 12 μl TE buffer and then ligated with 3 μl of equal mixture of phosphorylated adapters (100 μM) in a total volume of 20 μl at 4°C overnight (see ref. 13 for the sequence of the adapter). The adapters were annealed by heating for 2 min at 88°C, 10 min at 65°C, 10 min at 37°C, and 5 min at room temperature (17). After ligation, the samples were precipitated by the addition of spermine and washed as described (13). The adapted cDNA was resuspended in 20 μl of TE and electrophoresed on a 1% low melting temperature agarose gel. cDNAs of more than 570 bp in length were gel-purified for ligation into λ-ACT arms. The cDNA prepared from 0.5 μg of poly(A) RNA was ligated to 2 μg of T-filled λ-ACT plasmid DNA (11) in a volume of 5 μl at 4°C overnight and packaged using Gigapack Gold packaging extract (Stratagene). Automatic subcloning conversion of the Arabidopsis cDNA library into the plasmid library was performed as described (11). A total of 36 million independent recombinants were obtained. The phage library was amplified in E. coli LE392 and stored at −80°C in the presence of 7% dimethyl sulfoxide.
Yeast Transformation and Two-Hybrid Screening of the λ-ACT cDNA Library.
Yeast transformation was performed using the PEG/LiAcetate method as described (18). Yeast strain Y190 was first transformed with pGal4BD-IAA1 followed by transformation with the λ-ACT cDNA library. The transformants were plated on synthetic complete (SC) medium-Trp, Leu, His containing 25 mM 3-aminotriazole (3-AT) (Sigma) and incubated at 30°C for 6–7 days. 3-AT was used to repress the basal activity of the His3 reporter gene resulting in unspecific background growth in the absence of exogenous histidine (19). His+ colonies were assayed for β-gal activity by filter-lift assay (20) and His+,LacZ+ yeast cells were colony-purified. pACT cDNA plasmids were rescued into E. coli MH4 and cDNAs were identified by digestion analysis with XhoI. To eliminate the false positives, the rescued pACT cDNAs were retransformed into Y190 containing pGal4BD-IAA1 or pGal4BD and again tested for His3 and LacZ gene expression. The cDNAs were sequenced using Taq FS dye terminator chemistry (Applied Biosystems).
Expression and Purification of (His)6-Tagged PS-IAA4 and IAA1 Proteins.
The full-length cDNAs of PS-IAA4 and IAA1 generated by PCR were subcloned into pQE plasmids (Qiagen, Chatsworth, CA) to produce PS-IAA4 and IAA1 with C-terminal (His)6 tags. The recombinant proteins were expressed in E. coli M15[pREP] (Qiagen) at 30°C for 4 h by induction with 1.5 mM isopropyl β-d-thiogalactopyranoside. The bacterial pellets were extracted in 5 ml of ice-cold buffer [50 mM K2HPO4/KH2PO4, pH 8.0/1 M KCl/0.1 mM DTT/5 mM benzamidin⋅HCl/1 mM ɛ-aminocaproic acid/8 mM phenylmethylsulfonyl fluoride/0.1% (wt/vol) leupeptin and pepstatin] per g fresh weight with a French press. The extracts were cleared by centrifugation at 20000 × g for 30 min at 4°C. The supernatants were incubated with 125 μl of Ni-nitrilotriacetic acid (NTA) agarose (Qiagen) per ml at 4°C for 1.5 h. The Ni-NTA agarose was washed with buffer A (50 mM K2HPO4/KH2PO4, pH 8.0/1 M KCl/0.1 mM DTT/40 mM imidazole) until the flow-through at A280 was less than 0.02. The proteins were eluted with the buffer A containing 0.4 M imidazole.
Gel Filtration, Cross-Linking, SDS/PAGE, and Western Blot Analysis.
Gel-filtration was performed at 4°C at the flow rate of 0.5 ml/min with buffer containing 50 mM K2HPO4/KH2PO4, pH 8.0/1 M KCl/0.1 mM DTT using a Superdex 200 column (Pharmacia) connected to fast protein liquid chromatography. The column was calibrated with 250 μl of calibration standard solution containing chymotrypsinogen A (25 kDa), ovalbumin (43 kDa), BSA (67-kDa, monomer; 134-kDa dimer) according to the manufacturer’s instruction (Pharmacia) and run with 250 μl of Ni-NTA purified PS-IAA4 and IAA1 proteins (0.1 mg/ml each). For cross-linking experiments, Ni-NTA purified PS-IAA4 (0.1 mg/ml), IAA1 (0.1 mg/ml), or ribonuclease A (0.5 mg/ml) were incubated in buffer A with 0.5 mM bi(sulfosuccinimidyl)suberate (BS3; (Pierce) at 25°C for 1 min in a total volume of 50 μl. Control reactions were carried out without BS3. The reactions were terminated by adding 25 μl of SDS sample buffer. SDS/PAGE, Coomassie staining, Western blot analysis, and immunodetection of the proteins with a (His)6 tag-specific mAb (Dianova, Hamburg, Germany) were performed as described (21).
RESULTS
Homotypic Interactions of the Aux/IAA Gene Products in the Yeast Two-Hybrid System.
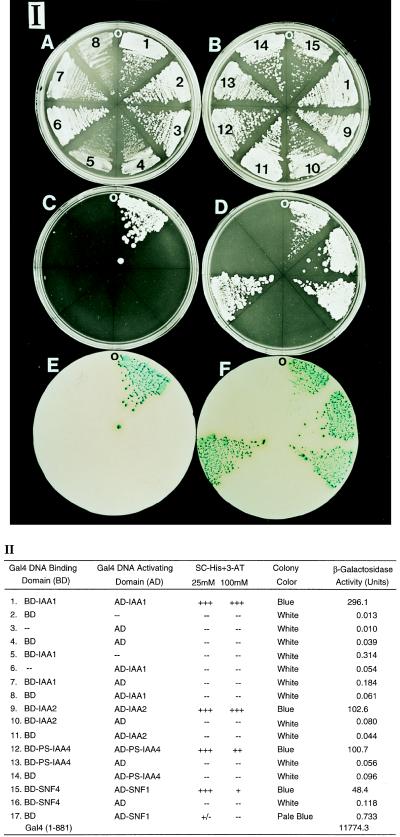
We used the yeast two-hybrid system (9) to study homotypic interactions between representative members of the Aux/IAA gene family. IAA1 and IAA2 from Arabidopsis and PS-IAA4 from pea were fused to the Gal4BD and Gal4AD, respectively, and transformed into the yeast reporter strain Y190 harboring two reporter genes, His3 and LacZ, integrated into the chromosome. SNF4 and SNF1 were used as a positive control (13). The His3 reporter activity was assayed by testing the growth of the transformants on SC medium lacking histidine and containing 3-AT (see Material and Methods). LacZ reporter gene expression was monitored by filter-lift assay and quantitative enzymatic assay of β-gal. As shown in Fig. 1 IC1 and II1, the yeast strain that contains the Gal4BD-IAA1 and Gal4AD-IAA1 constructs is able to grow on SC medium lacking histidine and containing 100 mM of 3-AT. None of the negative controls is able to grow on SC-His+3-AT (Fig. 1 IC2–8 and D10, 11, 13, 14 and II2–8, 10, 11, 13, 14). In addition, the color of the colonies in the yeast strain containing Gal4BD-IAA1 and Gal4AD-IAA1 is blue in the filter-lift assay (Fig. 1 IE1 and II1), whereas all of the negative controls fail to stain. Furthermore, the β-gal activity is 1,000- to 10,000-fold higher than the activity of the negative controls (Fig. 1 II1, IE2–8, D10, 11, 13, 14 and II2–8, 10, 11, 13, 14). The same results are found for IAA2 and PS-IAA4 (Fig. 1 ID9, 12 and F9, 12 and II9, 12) suggesting that IAA1, IAA2, and PS-IAA4 can perform homotypic interactions in yeast.
Figure 1.
Homotypic interactions of IAA1, IAA2, and PS-IAA4 in yeast. (I) Growth of yeast transformants on SC (A and B), SC-His containing 25 mM 3-AT (C and D), and the colony color of the transformants determined by the filter-lift assay (E and F). A, C, and E show the homotypic interaction of IAA1. B, D, and F show the homotypic interactions of IAA2 and PS-IAA4. The numbers shown on A and B correspond to the numbers on the left column of II. O indicates the orientation of each plate. (II) The relative growth, colony color, and β-gal activity of the yeast transformed with the indicated plasmids. Growth on SC-His plates containing 25 and 100 mM 3-AT, respectively, was scored as follows: +++, strong growth; ++, good growth; +, poor growth; +/−, very little growth; −, no growth. The color of the colonies was determined by the filter-lift assay, and β-gal activity was quantified enzymatically (see Material and Methods). The enzyme activities are the average of three independent yeast colonies.
Homodimerization of IAA1 and PS-IAA4 in Vitro.
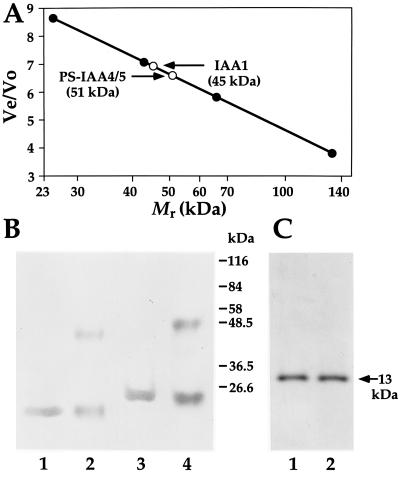
To test the physical association and the oligomerization of IAA1 and PS-IAA4, the corresponding (His)6-tagged proteins were expressed in E. coli, purified on an Ni-NTA column, and analyzed by gel filtration and chemical cross-linking. Fig. 2A shows the elution positions of IAA1-(His)6 and PS-IAA4-(His)6 after gel filtration on a Superdex 200 column precalibrated with molecular weight standards (see Materials and Methods). IAA1-(His)6 as well as PS-IAA4-(His)6 elute as single peaks of an apparent molecular weight of 45 kDa and 51 kDa, respectively, corresponding to their dimeric size. To confirm the gel filtration data the IAA1-(His)6 and PS-IAA4-(His)6 proteins were cross-linked with the noncleavable chemical cross-linker BS3. Cross-linked protein bands of the dimeric size appear when BS3 is present during incubation (Fig. 2B, compare lanes 2 and 4 with lanes 1 and 3, respectively). The monomeric protein ribonuclease A does not form any oligomeric structures in the presence of BS3 indicating the specificity of the cross-linking reaction for the Aux/IAA proteins (Fig. 2C, compare lane 2 with 1). These experiments are in agreement with the in vivo ones and suggest that IAA1-(His)6 and PS-IAA4-(His)6 physically interact and form stable homodimers.
Figure 2.
Homodimerization of IAA1 and PS-IAA4 in vitro. (A) Elution positions (○) of (His)6-tagged IAA1 and (His)6-tagged PS-IAA4 after gel filtration relative to those of the molecular weight standard (•). Ve/Vo, Elution volume/void volume. The x axis is in log scale. (B) Cross-linking of (His)6-tagged IAA1 and (His)6-tagged PS-IAA4. Ni-NTA purified (His)6-tagged IAA1 (lanes 1 and 2) and (His)6-tagged PS-IAA4 (lanes 3 and 4) were incubated in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of BS3 for 1 min at 25°C. After terminating the reactions, the proteins were run on SDS/PAGE gel, transferred to membrane, and immunodetected using a (His)6 tag-specific mAb. Numbers on the right of the blot indicate the size of the molecular weight standards. (C) Cross-linking of ribonuclease A. Ribonuclease A, a monomeric protein, was incubated with (lane 2) or without (lane 1) BS3, run on SDS/PAGE gel, and stained with Coomassie blue. The arrow indicates the position of ribonuclease A (molecular weight, 13 kDa).
Dimerization Domain Analysis of PS-IAA4.
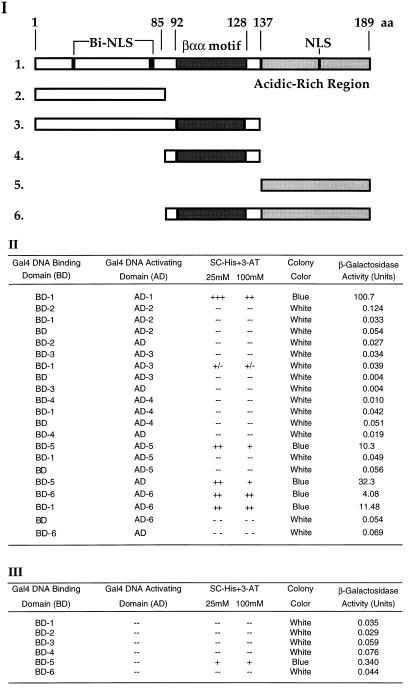
According to the structure of the βαα domain in the MetJ and Arc repressors of E. coli, the β-strands pair to form an antiparallel DNA-binding β-sheet. The second helix of the βαα domain is involved in dimerization whereas the first helix mediates cooperative binding of dimer units (8). The putative βαα domain of the Aux/IAA proteins may also be responsible for their in vivo and in vitro dimerization properties. To test this possibility, we constructed different deletions of PS-IAA4 (Fig. 3I) and analyzed them in the yeast two-hybrid system. As shown in Fig. 3II, the βαα motif alone (peptide 4)and the N-terminal peptide containing the βαα motif (peptide 3) do not show any reporter gene activity. In contrast, the C-terminal peptide containing the βαα motif (peptide 6) is capable of interacting with itself as well as with the full-length protein. The C-terminal peptide without the βαα domain (peptide 5) also is capable of reporter gene expression. However, this activity is not due to specific protein–protein interaction but to intrinsic transcriptional activity which seems to be masked in the full-length PS-IAA4 protein (Fig. 3 II and III). To exclude the possibility that the differential lack of reporter gene activity may be due to differential stability of the tested peptides in yeast we carried out immunoprecipitation and Western blot analyses using yeast crude extracts and a polyclonal antibody against the Gal4BD. Although the expression of full-length PS-IAA4 as well as of peptides 5 and 6 could easily be demonstrated, neither the βαα peptide 4 nor peptide 3 (data not shown) could be detected. Thus, although the βαα domain is present in all interacting peptides, we cannot conclude from the data of Fig. 3 that this domain is necessary and sufficient for the dimerization of PS-IAA4 (see Discussion).
Figure 3.
Interaction domain analysis of PS-IAA4 in the yeast two-hybrid system. (I) Schematic diagram showing the deletion constructs of PS-IAA4 (constructs 1–6). The numbers indicate the positions of the deletions (constructs 2–6) with regard to full-length PS-IAA4 (construct 1). The DNA sequences encoding the different peptides were fused in-frame to the DNA binding domain and transactivation domain of Gal4, respectively, and the constructs were transformed into Y190. Cells were grown on SC-His plates containing 25 and 100 mM 3-AT, respectively, and assayed for colony color and β-gal activity. (II and III) The tables show the relative growth, colony color, and β-gal activity of yeast cells containing the indicated plasmids.
Heterotypic Interaction of IAA1, IAA2, and PS-IAA4.
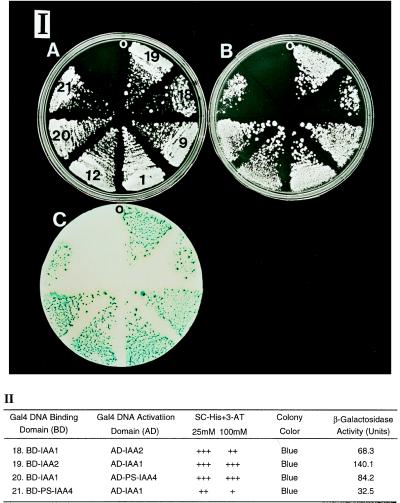
Because all the characterized Aux/IAA gene products contain the postulated βαα motif (22), we tested putative heterotypic interactions of IAA1, IAA2, and PS-IAA4 in the yeast two-hybrid system. These studies show that heterotypic interactions occur between IAA1 and IAA2 as well as between IAA1 and PS-IAA4 (Fig. 4). When the cDNA fusion is switched from the Gal4BD to Gal4AD or vice versa, the His3 and LacZ reporters are still activated. These results using such reciprocal hybrid proteins indicate that reporters reflect the physical associations between different AUX/IAA proteins (23).
Figure 4.
Heterotypic interaction of IAA1 with IAA2 and PS-IAA4. (I) Growth of yeast cells transformed with the indicated plasmids on SC plates (A), SC-His plates containing 25 and 100 mM 3-AT, respectively (B), and the colony color of the transformants determined by filter-lift assay (C). The numbers shown on A correspond to the numbers of the plasmids shown on the left column of II in this figure and in Fig. 1. (II) The table shows the relative growth, colony color, and β-gal activity of the yeast cells transformed with the indicated plasmids.
Generation of a λ-ACT Gal4AD-Tagged cDNA Library from Arabidopsis and Two-Hybrid Screening Using IAA1 As Bait.
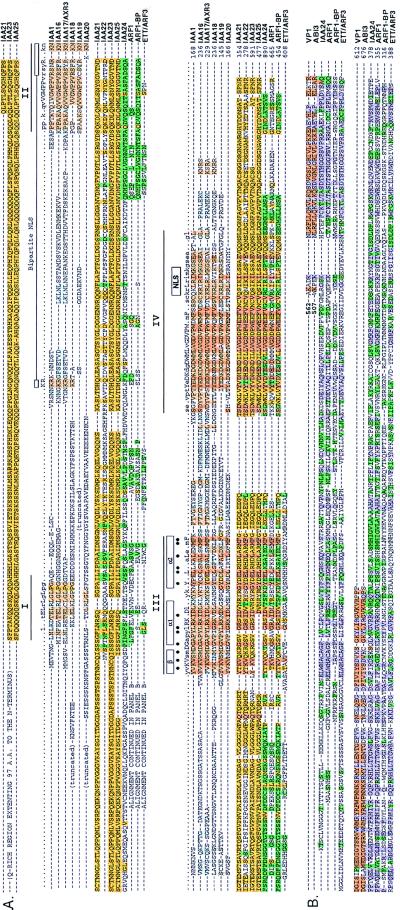
To explore the possibility that novel proteins may interact with the Aux/IAA gene products and therefore might be involved in auxin signaling, a Gal4 transactivation domain-tagged cDNA library from 3-day-old etiolated Arabidopsis seedling was constructed using the λ-ACT system. Because the λ-ACT system utilizes cre–lox-mediated site-specific recombination to convert the Gal4AD DNA from phage to plasmid form, it allows the generation of large cDNA libraries with a high percentage of inserts (16). Our Arabidopsis library contains 3.6 × 107 recombinants with 95% of the clones containing inserts. The library was amplified, converted to plasmid form, and used for a yeast two-hybrid screening with the IAA1 as a “bait.” From a total of 3.6 million transformants, 123 His+-LacZ+ clones were obtained. Among the 123 clones, 89 pACT cDNA plasmids were rescued in E. coli. After retransformation of the pACT cDNA plasmids into yeast reporter strain containing Gal4BD-IAA1 or Gal4BD, and again monitoring for the His+-LacZ+ phenotype, 85 clones were considered true positives and sequenced. Table 1 shows the isolated cDNAs can be classified based on their sequences into two groups. Group I contains previously identified IAA genes, such as IAA2, IAA3, IAA4, IAA8, and IAA9. All these cDNAs were independently isolated multiple times and contain different 5′ ends. The detection of IAA2, 3, 4, 8, and 9 with IAA1 as a bait strongly indicates that heterotypic interactions among the early auxin-induced Aux/IAA gene products are prevalent in Arabidopsis. Group II contains 10 previously unidentified Aux/IAA-like cDNAs, named IAA16–25. Fig. 5A shows their amino acid alignment compared with that of IAA1, a “classical” Aux/IAA protein. The recent discovery that mutations in domain II of IAA17 are responsible for the AXR3 mutant (O. Leyser, personal communication) offers genetic evidence for the functional significance of the AUX/IAA proteins in auxin signaling. The IAA16–19 proteins contain all the structural and functional elements described for the classical Aux/IAA proteins (23), except for IAA20, which does not have domain II [bipartite nuclear localization signal (NLS)] and has a less-conserved simian virus 40-type NLS. IAA21–25 are larger and highly divergent in the N terminus compared with the other Aux/IAA proteins. The N-terminal conserved domains I and II are absent and their N termini do not have any homology to the Aux/IAA proteins. Furthermore, both NLSs are missing and the putative βαα motif is present in all of them except for IAA25 where the β-sheet is absent (Fig. 5A). The N termini of the IAA23–25 proteins are rich in glutamic acid. Sequence alignment shows that IAA21, 23, and 24 are quite similar in the N terminus (Fig. 5A) and may constitute members of a new class of transcriptional regulators. The absence of the glutamate-rich domain in IAA21 may be due to a truncated cDNA. The N terminus of IAA22 is quite different from all the newly discovered members and may also be a member of another class of transcription factors. It remains to be determined whether these newly discovered Aux/IAA-like gene products IAA16–25 are nuclear localized and auxin-regulated. Recently, it was discovered that the IAA24 protein shares extensive amino acid identity with the auxin response factor 1 (ARF1) (Fig. 5 A and B), a transcription regulator that binds to an early auxin responsive element (37). In addition, IAA24 has amino acid identity with the N termini of the ARF1, ARF1-BP, and ETT/ARF3 gene products (37, 38). The C terminus of IAA24 is similar to the C termini of ARF1 and its binding protein ARF1-BP where the βαα containing domains III and IV are located (Fig. 5A). In addition, the N terminus of IAA24 is similar to the N termini of the βαα-containing ARF1 and ARF1-BP proteins and also to the non-βαα containing ETT/ARF3 protein (ref. 38; Fig. 5B). This N-terminal region is similar to the C terminus of the maize transcriptional activator Viviparous-1 (VP1; ref. 39) and its Arabidopsis homolog ABI3 (40). Thus, IAA24, ARF1, and ARF1-BP proteins consist of a new class of transcription factors that contain the prokaryotic βαα and the eukaryotic VP1/ABI3 motifs. The ETT/ARF3 protein is responsible for patterning Arabidopsis floral meristems and reproductive organs (38).
Table 1.
Proteins encoded by the cDNAs isolated from the yeast two-hybrid screening using IAA1 as bait
| Group | cDNA | No. of clones* | GenBank accession no. | Colony color
|
|
|---|---|---|---|---|---|
| BD-IAA1 | BD | ||||
| Group I | IAA2 | 3 | L15449 | Blue | White |
| IAA3 | 6 | U18406 | Blue | White | |
| IAA4 | 8 | L15450 | Blue | White | |
| IAA8 | 7 | U18410 | Blue | White | |
| IAA9 | 2 | U18411 | Blue | White | |
| Group II | IAA16 | 20 | U49072 | Blue | White |
| IAA17 | 16 | U49073 | Blue | White | |
| IAA18 | 2 | U49074 | Blue | White | |
| IAA19 | 1 | U49075 | Blue | White | |
| IAA20 | 11 | U49076 | Blue | White | |
| IAA21 | 2 | U49077 | Blue | White | |
| IAA22 | 4 | U53672 | Blue | White | |
| IAA23 | 1 | U79555 | Blue | White | |
| IAA24 | 1 | U79557 | Blue | White | |
| IAA25 | 1 | U79556 | Blue | White | |
The λ-ACT Arabidopsis cDNA library was screened with IAA1 as bait using the two-hybrid system. Plasmids of clones which gave a His+ and LacZ+ phenotype were retransformed into yeast strains containing either Gal4BD-IAA1 or Gal4BD and tested again for His3 and LacZ reporter gene expression. The cDNAs of constructs which produced a His+ and LacZ+ phenotype with BD-IAA1 but not with Gal4BD were sequenced and classified into two groups. Group I includes known Aux/IAA homologues and group II includes previously unidentified members of the Aux/IAA gene family. IAA17 is identical to the F19P19.31 gene recently identified by genomic sequencing of BAC F19P9 (GenBank accession no. AC0000104).
Number of independently isolated clones encoding identical Aux/IAA polypeptides.
Figure 5.
(A) Sequence alignment and domain structure of Aux/IAA proteins isolated by two-hybrid screening compared with those of ARF1, ARF1-BP, and ETT/ARF3 (37, 38). Identical and conserved amino acid residues (at least 50% matches) are shaded (light brown) and appear in the consensus (capital and small letters, respectively). Conserved domains are underlined and indicated by Roman numerals. Amino acid residues conserved within a distinct lineage are colored. Amino acid residues that may form hydrophobic surfaces in the predicted conserved amphipathic βαα motif are indicated by (•). NLS indicates the nuclear localization signal. The numbers at the right of the sequences indicate the number of amino acid residues for the corresponding coding region of the cDNA. The consensus sequence shown at the top of the alignment was obtained from the IAA1–15 proteins (34). IAA1, the prototype Aux/IAA protein, is shown below the consensus. (B) Similarity of the N-terminal region of IAA24, ARF1, ARF1-BP, and ETT/ARF3 with a portion of the C-terminal region of VP1 (39) and ABI3 (40).
DISCUSSION
In this communication we describe the homo- and heterotypic interactions of Aux/IAA proteins from Arabidopsis and pea in yeast. Cross-linking and gel filtration experiments also demonstrate the formation of stable dimers in vitro suggesting that these proteins may also form dimers in planta. The deletion analysis of PS-IAA4 as well as the structure of the Aux/IAA cDNAs obtained by the two-hybrid screen indicate that the postulated βαα domain is a prerequisite for their stable dimer formation. Due to the instability of some of the tested fusion peptides in yeast, it is not clear whether the βαα domain is sufficient for proper in vivo dimerization. However, gel filtration experiments performed with a set of purified recombinant (His)6-tagged PS-IAA4 peptides similar to those tested in yeast reveals that the putative βαα domain is responsible for stable in vitro dimerization (K.H. and A.T., unpublished data). These results are in agreement with our proposition that the βαα domain of the Aux/IAA gene products is structurally related to that of the dimeric prokaryotic transcriptional repressors MetJ and Arc (8). Furthermore, because IAA24 lacks the β-sheet motif and a substantial portion of the α1 helical region, but still is capable of forming a dimer with IAA1, the α2 helical region of the βαα domain may be critical for dimerization. This finding is consistent with the crystal structures of MetJ and Arc where the α2 helix from each monomer packs against each other and forms direct contacts in the dimer. In addition, in vitro and in vivo binding site selection assays reveals that the full-length IAA1 protein and its βαα domain have sequence-specific DNA-binding activity (S. Abel and A.T., unpublished data). PS-IAA4 also contains a C-terminal acidic domain that is able to transactivate the His3 and LacZ reporter genes when fused to the Gal4 DNA-binding domain (Fig. 3). However, this activity is masked when the intact protein is expressed in yeast (Fig. 3). These data strongly indicate that the nuclear localized Aux/IAA gene products may be transcriptional regulators capable of forming homo- and heterodimers.
The finding that the Aux/IAA gene family is comprised of homo- or heterodimeric transcription factors has important implications for their putative role in auxin signal transduction. Intensive studies of the basic leucine zipper (bZIP), basic helix-loop-helix (bHLH), and the ligand-dependent nuclear transcription factor families suggest that dimerization between different members can provide an enormous repertoire of different regulatory proteins (24–26). First, homo- and heterodimeric complexes between different polypeptides can bind with significantly different efficiency to DNA (27). Second, dimer formation can influence the DNA-binding specificity of homo- or heterodimeric complexes (25, 28). Third, a missing or altered DNA-binding domain in one of the polypeptides of the heterodimeric complex can produce a dominant negative regulator (29–31). Fourth, homo- and heterodimeric complexes can have different affinities to corepressors or cotransactivators (32). Our results suggest a similar regulatory potential by dimerization/heterodimerization of the Aux/IAA proteins, though these need to be confirmed by in planta studies. The possibility exists that the Q-rich region of IAA23–25 may be involved in direct protein interaction with the basal transcriptional machinery allowing them to act as strong transcriptional activators in response to auxin for certain subsets of late genes. This proposition is supported by the recent finding that IAA24 is similar to ARF1, a transcriptional regulator that binds to an early auxin responsive element (37).
The large size of the Aux/IAA gene family (at least 25 members) and the ability of its members to homo- and heterodimerize constitutes a vast potential for regulation of late or downstream genes. This proposition is supported by a simple calculation using the formula, C = n(n + 1)/2!, where n = 25 (the number of different Aux/IAA proteins) and C = the number of dimers. It indicates that 325 different homo- and heterodimers can be formed. If the Aux/IAA products bind to DNA as tetramers like the Arc family of prokaryotic repressors (8), then 20,475 different tetramers can be formed as indicated by the formula, C = n(n + 1)(n + 2)(n + 3)/4!. In addition, based on their differential mode of expression with respect to timing, hormone concentration, and spatially restricted expression pattern (33–36), we propose that tissue- and cell-specific subsets of homo- and heterodimeric Aux/IAA transcription factors are responsible for the differential gene expression pattern of late auxin-regulated genes. These interactions therefore establish the highly pleiotropic and complex physiological and morphological auxin responses during plant development.
Acknowledgments
We thank Dr. S. Elledge for providing us with λ-ACT and Dr. S. Fields for pGBT9.BS and pGAD.GH. We also thank Dr. S. Abel for useful discussions, providing us with pQE-PS-IAA4 and pQE-IAA1 plasmids and sharing some of his unpublished data. The help of Dr. P. Overvoorde with sequence alignments is greatly appreciated. The assistance of Ms. Guixia Yu in figure preparation is also acknowledged. This research was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft (Ha 2146/2-1), Germany to K.H. and by a grant from the National Institutes of Health (GM-35447) to A.T.
ABBREVIATIONS
- IAA
indoleacetic acid
- aa
amino acid
- 3-AT
3-aminotriazole
- SC
synthetic complete medium
- Gal4BD
Gal4 DNA binding domain
- Gal4AD
Gal4 activation domain
- BS3
Bi(sulfosuccinimidyl)suberate
- NTA
nitrilotriacetic acid
- β-gal
β-galactosidase
References
- 1.Went F W, Thimann K V. Phytohormones. New York: Macmillan; 1937. [Google Scholar]
- 2.Estelle M. BioAssays. 1992;14:439–443. doi: 10.1002/bies.950140703. [DOI] [PubMed] [Google Scholar]
- 3.Theologis A, Huynh T V, Davis R W. J Mol Biol. 1985;183:53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- 4.Theologis A. Annu Rev Plant Physiol. 1986;37:407–438. [Google Scholar]
- 5.Guilfoyle T J. CRC Crit Rev Plant Sci. 1986;4:247–276. [Google Scholar]
- 6.Abel S, Theologis A. Plant Physiol. 1996;111:9–17. doi: 10.1104/pp.111.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abel S, Oeller P W, Theologis A. Proc Natl Acad Sci USA. 1994;91:326–330. doi: 10.1073/pnas.91.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pabo C O, Saur R T. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- 9.Fields S, Song O-K. Nature (London) 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 10.Hall M N, Hereford L, Herskowitz I. Cell. 1984;36:1057–1065. doi: 10.1016/0092-8674(84)90055-2. [DOI] [PubMed] [Google Scholar]
- 11.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 12.Kieber J J, Rothenberg M, Roman G, Feldmann K A, Ecker J R. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- 13.Elledge S J, Mulligan J T, Ramer S W, Spottswood M S, Davis R W. Proc Natl Acad Sci USA. 1991;88:1731–1735. doi: 10.1073/pnas.88.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarente L. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- 15.Iwabuchi K, Li B, Bartel P L, Fields S. Oncogene. 1993;8:1693–1696. [PubMed] [Google Scholar]
- 16.Durfee T D, Becherer K, Chen P-L, Yeh S-H, Yang Y, Kilburn A E, Lee W-H, Elledge S J. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 17.Kadonaga J T, Tjian R. Proc Natl Acad Sci USA. 1986;83:5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gietz D, St. Jean A, Woods R A, Schiesh R H. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishore G M, Shah D M. Annu Rev Biochem. 1988;57:627–663. doi: 10.1146/annurev.bi.57.070188.003211. [DOI] [PubMed] [Google Scholar]
- 20.Breeden L, Nasmyth K. Cold Spring Harbor Symp Quant Biol. 1985;50:643–650. doi: 10.1101/sqb.1985.050.01.078. [DOI] [PubMed] [Google Scholar]
- 21.Harter K, Talke-Messerer C, Barz W, Schäfer E. Plant J. 1993;4:507–516. [Google Scholar]
- 22.Abel S, Nguyen M D, Theologis A. J Mol Biol. 1995;251:533–549. doi: 10.1006/jmbi.1995.0454. [DOI] [PubMed] [Google Scholar]
- 23.Estojak J, Brent R, Golemis E A. Mol Cell Biol. 1995;15:5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones N. Cell. 1990;61:9–11. doi: 10.1016/0092-8674(90)90207-u. [DOI] [PubMed] [Google Scholar]
- 25.Menkens A E, Schindler U, Cashmore A R. Trends Biochem. 1995;20:506–510. doi: 10.1016/s0968-0004(00)89118-5. [DOI] [PubMed] [Google Scholar]
- 26.Thummel C S. Cell. 1995;83:871–877. doi: 10.1016/0092-8674(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 27.Murre C, McCaw P S, Vaessin H, Caudy M, Jan L Y, Jan Y N, Cabrera C V, Buskin J N, Huaschka S D, Lassar A B, Weintraub H, Baltimore D. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 28.Hai T, Curran T. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benezra R, Davis R, Lockshon D, Turner D L, Weintraub Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 30.Ellis H M, Spann D R, Posakony J W. Cell. 1990;61:27–38. doi: 10.1016/0092-8674(90)90212-w. [DOI] [PubMed] [Google Scholar]
- 31.Garrell J, Modolell J. Cell. 1990;61:39–48. doi: 10.1016/0092-8674(90)90213-x. [DOI] [PubMed] [Google Scholar]
- 32.Hörlein A J, Näär A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass C K, Rosenfeld M G. Nature (London) 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 33.Wyatt R E, Ainley W M, Nagao R T, Conner T W, Key J L. Plant Mol Biol. 1993;22:731–749. doi: 10.1007/BF00027361. [DOI] [PubMed] [Google Scholar]
- 34.Abel S, Nguyen D, Theologis A. J Biol Chem. 1995;270:533–549. doi: 10.1074/jbc.270.32.19093. [DOI] [PubMed] [Google Scholar]
- 35.Koshiba T, Ballas N, Wong L M, Theologis A. J Mol Biol. 1995;253:396–413. doi: 10.1006/jmbi.1995.0562. [DOI] [PubMed] [Google Scholar]
- 36.Wong L M, Abel S, Shen N, de la Foata M, Mall Y, Theologis A. Plant J. 1996;9:587–599. doi: 10.1046/j.1365-313x.1996.9050587.x. [DOI] [PubMed] [Google Scholar]
- 37.Ulmasov T, Hagen G, Guilfoyle T J. Science. 1997;276:1865–1868. doi: 10.1126/science.276.5320.1865. [DOI] [PubMed] [Google Scholar]
- 38.Sessions, A. R., Nenhauser, J. L., McColl, A., Roe, J. L., Feldmann, K. A. & Zambryski, P. C. (1997) Development (Cambridge, U.K.), in press. [DOI] [PubMed]
- 39.McCarty D R, Hattori T, Carson C B, Vasil V, Lazar M, Vasil I K. Cell. 1991;66:895–905. doi: 10.1016/0092-8674(91)90436-3. [DOI] [PubMed] [Google Scholar]
- 40.Giraudat J, Hauge B M, Valon C, Smalle J, Parcy F, Goodman H M. Plant Cell. 1992;4:1251–1261. doi: 10.1105/tpc.4.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]