Abstract
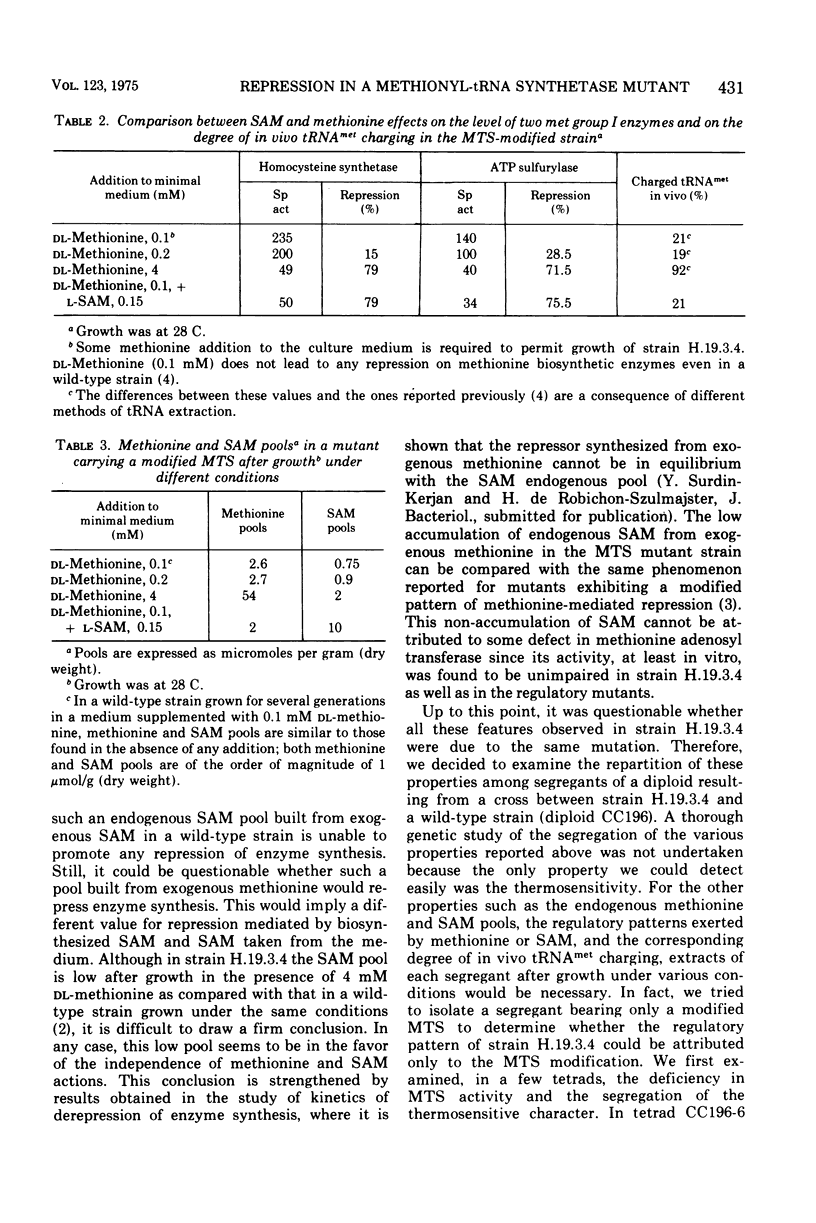
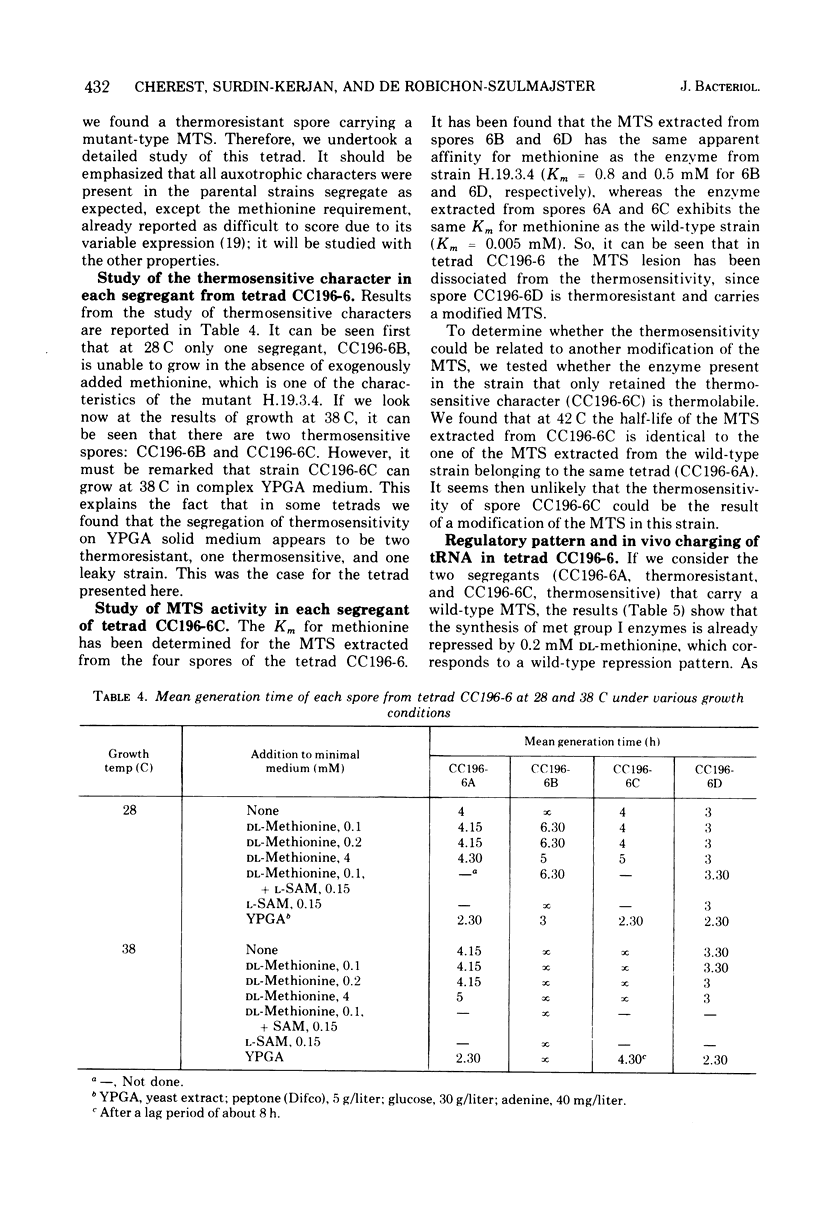
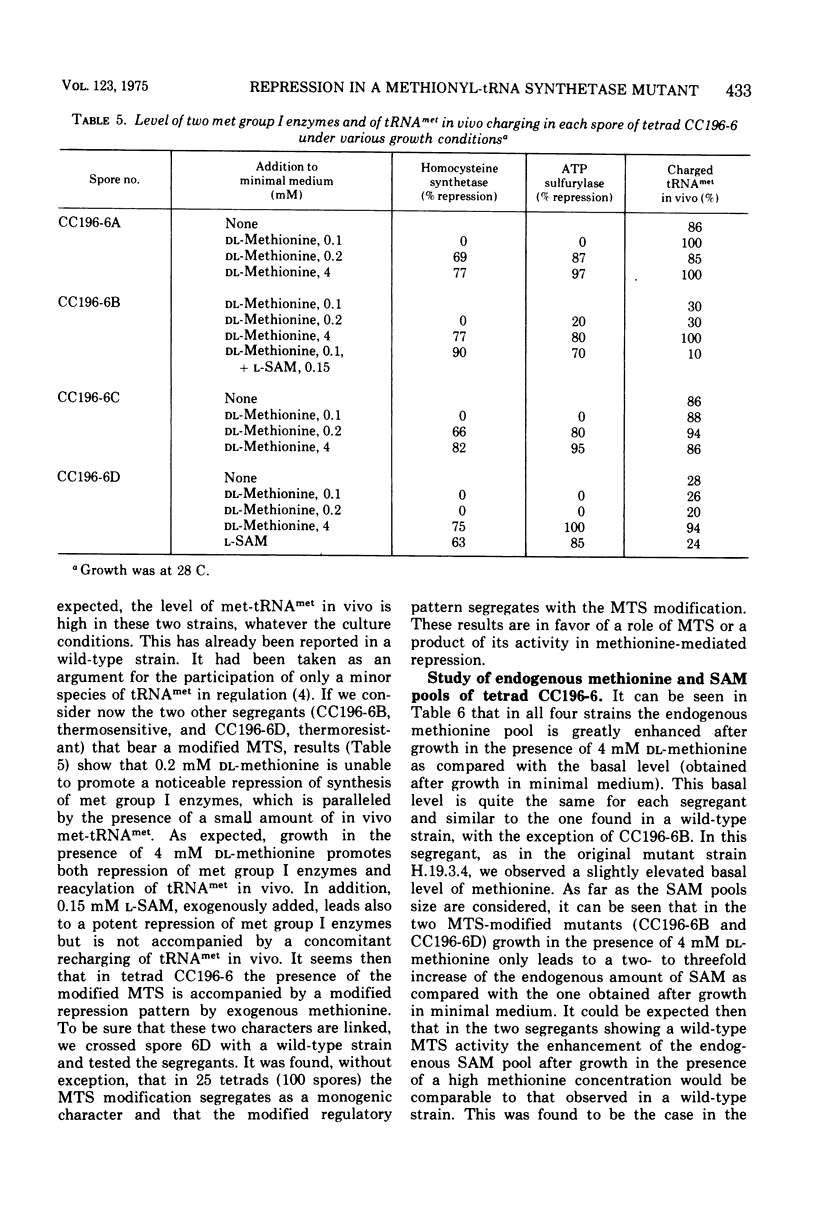
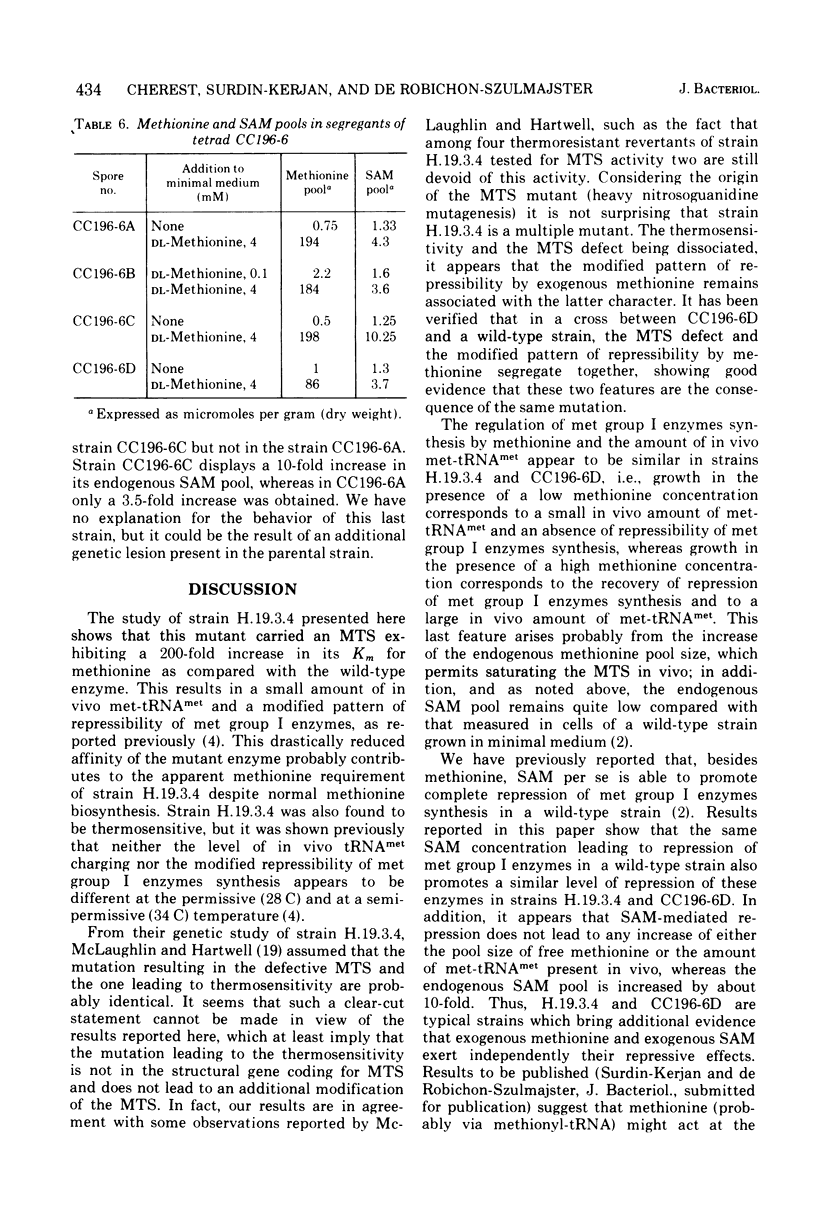
A Saccharomyces cerevisiae mutant strain unable to grow at 38 C and bearing a modified methionyl-transfer ribonucleic acid (tRNA) synthetase has been studied. It has been shown that, in this mutant, the percentage of tRNAmet charged in vivo paralleled the degree of repressibility of methionine biosynthetic enzymes by exogenous methionine. On the contrary, the repression mediated by exogenous S-adenosylmethionine does not correlate with complete acylation of tRNAmet. Althought McLaughlin and Hartwell reported previously that the thermosensitivity and the defect in the methionyl-tRNA synthetase were due to the same genetic lesion (1969), no diffenence could be found in the methionyl-tRNA synthetase activity or in the pattern of repressibility of methionine biosynthetic pathway after growth at the premissive and at a semipermissive temperature. It appears that the mutant also exhibits some other modified characters that render unlikely the existence of only one genetic lesion in this strain. A genetic study of this mutant was undertaken which led to the conclusion that the thermosensitivity and the other defects are not related to the methionyl-tRNA synthetase modification. It was shown that the modified repressibility of methionine biosynthetic enzymes by methionine and the lack of acylation of tRNAmet in vivo follow the methionyl-tRNA synthetase modification. These results are in favor of the idea that methionyl-tRNAmet, more likely than methionine, is implicated in the regulation of the biosynthesis of methionine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. Mechanism of repression of methionine biosynthesis in Escherichia coli. I. The role of methionine, s-adenosylmethionine, and methionyl-transfer ribonucleic acid in repression. Mol Gen Genet. 1973 Jul 16;123(4):299–324. doi: 10.1007/BF00433648. [DOI] [PubMed] [Google Scholar]
- Cherest H., Surdin-Kerjan Y., Antoniewski J., Robichon-Szulmajster H. S-adenosyl methionine-mediated repression of methionine biosynthetic enzymes in Saccharomyces cerevisiae. J Bacteriol. 1973 Jun;114(3):928–933. doi: 10.1128/jb.114.3.928-933.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H., Surdin-Kerjan Y., Antoniewski J., de Robichon-Szulmajster H. Effects of regulatory mutations upon methionine biosynthesis in Saccharomyces cerevisiae: loci eth2-eth3-eth10. J Bacteriol. 1973 Sep;115(3):1084–1093. doi: 10.1128/jb.115.3.1084-1093.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H., Surdin-Kerjan Y., Robichon-Szulmajster H. Methionine-mediated repression in Saccharomyces cerevisiae: a pleiotropic regulatory system involving methionyl transfer ribonucleic acid and the product of gene eth2. J Bacteriol. 1971 Jun;106(3):758–772. doi: 10.1128/jb.106.3.758-772.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVITO P. C., DREYFUSS J. METABOLIC REGULATION OF ADENOSINE TRIPHOSPHATE SULFURYLASE IN YEAST. J Bacteriol. 1964 Nov;88:1341–1348. doi: 10.1128/jb.88.5.1341-1348.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann B., Imbault P., Weil J. H. Determination of the degree of in vivo tRNA aminoacylation in yeast cells. Anal Biochem. 1974 Oct;61(2):548–556. doi: 10.1016/0003-2697(74)90423-0. [DOI] [PubMed] [Google Scholar]
- Ferro A. J., Spence K. D. Induction and repression in the S-adenosylmethionine and methionine biosynthetic systems of Saccharomyces cerevisiae. J Bacteriol. 1973 Nov;116(2):812–817. doi: 10.1128/jb.116.2.812-817.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALZY P., SLONIMSKI P. P. Evolution de la constitution enzymatique de la levure cultivée sur acide lactique ou sur glucose comme seule source de carbone. C R Hebd Seances Acad Sci. 1957 Dec 23;245(26):2556–2558. [PubMed] [Google Scholar]
- Greene R. C., Hunter J. S., Coch E. H. Properties of metK mutants of Escherichia coli K-12. J Bacteriol. 1973 Jul;115(1):57–67. doi: 10.1128/jb.115.1.57-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Su C. H., Holloway C. T. S-Adenosylmethionine synthetase deficient mutants of Escherichia coli K-12 with impaired control of methionine biosynthesis. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1120–1126. doi: 10.1016/0006-291x(70)90355-4. [DOI] [PubMed] [Google Scholar]
- Gross T. S., Rowbury R. J. Biochemical and physiological properties of methionyl-sRNA synthetase mutants of Salmonella typhimurium. J Gen Microbiol. 1971 Jan;65(1):5–21. doi: 10.1099/00221287-65-1-5. [DOI] [PubMed] [Google Scholar]
- Gross T. S., Rowbury R. J. Methionyl transfer RNA synthetase mutants of Salmonella typhimurium which have normal control of the methionine biosynthetic enzymes. Biochim Biophys Acta. 1969 Jun 17;184(1):233–236. doi: 10.1016/0304-4165(69)90126-3. [DOI] [PubMed] [Google Scholar]
- Hobson A. C., Smith D. A. S-adenosylmethionine synthetase in methionine regulatory mutants of Salmonella typhimurium. Mol Gen Genet. 1973 Oct 16;126(1):7–18. doi: 10.1007/BF00333477. [DOI] [PubMed] [Google Scholar]
- JOHNSTON J. R., MORTIMER R. K. Use of snail digestive juice in isolation of yeast spore tetrads. J Bacteriol. 1959 Aug;78:292–292. doi: 10.1128/jb.78.2.292-292.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- McClary D. O., Nulty W. L., Miller G. R. EFFECT OF POTASSIUM VERSUS SODIUM IN THE SPORULATION OF SACCHAROMYCES. J Bacteriol. 1959 Sep;78(3):362–368. doi: 10.1128/jb.78.3.362-368.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. S., Hartwell L. H. A mutant of yeast with a defective methionyl-tRNA synthetase. Genetics. 1969 Mar;61(3):557–566. doi: 10.1093/genetics/61.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszewski A., Grabski J. Regulation of S-amino acids biosynthesis in Aspergillus nidulans. Role of cysteine and-or homocysteine as regulatory effectors. Mol Gen Genet. 1974;132(4):307–320. doi: 10.1007/BF00268571. [DOI] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Résistance a l'éthionine chez Saccharomyces cerevisiae. II. Etude physiologique. Genetics. 1966 Oct;54(4):993–1006. doi: 10.1093/genetics/54.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLENK F., DEPALMA R. E. The formation of S-adenosylmethionine in yeast. J Biol Chem. 1957 Dec;229(2):1037–1050. [PubMed] [Google Scholar]
- Surdin-Kerjan Y., Cherest H., Robichon-Szulmajster H. Relationship between methionyl transfer ribonucleic acid cellular content and synthesis of methionine enzymes in Saccharomyces cerevisiae. J Bacteriol. 1973 Mar;113(3):1156–1160. doi: 10.1128/jb.113.3.1156-1160.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- Wiebers J. L., Garner H. R. Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast, and Escherichia coli. J Biol Chem. 1967 Dec 10;242(23):5644–5649. [PubMed] [Google Scholar]



