Abstract
Neurons undergoing targeted photolytic cell death degenerate by apoptosis. Clonal, multipotent neural precursor cells were transplanted into regions of adult mouse neocortex undergoing selective degeneration of layer II/III pyramidal neurons via targeted photolysis. These precursors integrated into the regions of selective neuronal death; 15 ± 7% differentiated into neurons with many characteristics of the degenerated pyramidal neurons. They extended axons and dendrites and established afferent synaptic contacts. In intact and kainic acid-lesioned control adult neocortex, transplanted precursors differentiated exclusively into glia. These results suggest that the microenvironmental alterations produced by this synchronous apoptotic neuronal degeneration in adult neocortex induced multipotent neural precursors to undergo neuronal differentiation which ordinarily occurs only during embryonic corticogenesis. Studying the effects of this defined microenvironmental perturbation on the differentiation of clonal neural precursors may facilitate identification of factors involved in commitment and differentiation during normal development. Because photolytic degeneration simulates some mechanisms underlying apoptotic neurodegenerative diseases, these results also suggest the possibility of neural precursor transplantation as a potential cell replacement or molecular support therapy for some diseases of neocortex, even in the adult.
Keywords: transplantation, stem cell, cell death, development, neurodegeneration
Neural cell replacement might offer a useful future therapy for some neurodegenerative diseases (1). The feasibility of using multipotent neural precursors to replace diseased neurons was tested in adult mouse neocortex with experimentally induced neurodegeneration using the approach of targeted photolytic neuronal degeneration (2–6).
Targeted neuronal degeneration provides a highly controllable model of apoptotic neuronal cell death in adult mammalian neocortex (4, 5). Neuronal degeneration that is spatially defined and cell population-specific can be produced by noninvasive photoactivation of retrogradely transported nanospheres carrying the photoactive chromophore chlorin e6 within targeted pyramidal neurons in lamina II/III with trans-callosal projections. This result is effected by using near-infrared laser illumination with beam-controlling optics to limit penetration to lamina II/III. There is no injury to nontargeted, intermixed neurons, glia, axons, or connective tissue (2, 3). Targeted pyramidal neurons undergo apoptotic degeneration that is slowly progressive and noninflammatory, reaching a peak 8–10 days following induction of cell death, and continuing over 3–4 weeks (3–5). The cell death is protein synthesis-dependent and associated with a cascade of apoptotic events initiated by singlet oxygen production (5).
Apoptosis is postulated to play a role in various neurodegenerative diseases including Alzheimer disease, Huntington disease, AIDS encephalopathy, amyotrophic lateral sclerosis, and some aspects of ischemia (reviewed in ref. 7). It is also known to be pivotal in normal central nervous system (CNS) development (7). Previous results suggest that signals involved in directed migration, differentiation, and integration normally seen during fetal corticogenesis can be re-expressed in postdevelopmental mouse neocortex if highly selective and noninflammatory apoptotic neuronal death is induced synchronously by targeted photolytic degeneration (3, 4). This process may recapitulate signals modulated by programmed cell death during normal development, to which multipotent neural precursors might respond.
A number of recent studies (reviewed in ref. 8) demonstrate that neural precursors can integrate into normal host rodent brain in a cytoarchitecturally correct manner and differentiate into neurons and/or glia in a manner appropriate to the site of engraftment and the developmental stage of the recipient (8–18). These data suggest that such neural precursors might be used therapeutically to replace lost neural cell types. We tested whether multipotent precursors could respond to the specifically perturbed microenvironment present during targeted apoptotic cell death in the adult mouse neocortex and undergo directed neuronal differentiation.
C17.2 is a clonal multipotent neural precursor cell line originally derived from the external germinal layer of neonatal mouse cerebellum (15–17). These cells can participate in the normal development of cerebellum and many other structures, including embryonic neocortex, upon implantation into mouse germinal zones. C17.2 cells can differentiate into pyramidal neurons when transplanted into the mid-embryonic mouse brain during normal corticogenesis, but do not undergo neuronal differentiation at later developmental stages when neurogenesis has normally ceased and gliogenesis predominates (16, 17).
We found that transplanted C17.2 multipotent precursors differentiated into neurons in adult mouse neocortex uniquely within regions of targeted apoptotic neuronal degeneration, by morphologic, ultrastructural, and immunocytochemical analysis. These results suggest (i) the potential of neural precursor transplantation as a possible therapy for some neurodegenerative diseases (18), and (ii) the use of this microenvironmental perturbation to study neuronal commitment and differentiation in vivo.
MATERIALS AND METHODS
Targeted Photolytic Apoptosis and Precursor Cell Transplantation.
C57B/6J mice were used in these experiments (n = 55). The experimental protocol is summarized schematically in Fig. 1. Targeted apoptotic degeneration of layer II/III pyramidal neurons was initiated within an optically delimited region of the neocortex in one hemisphere in 4-week-old mice (n = 25) as described (3). Approximately 70% of such selectively targeted neurons are eliminated within spatially defined regions of adult neocortex (3). At 6 weeks of age, during active degeneration of targeted pyramidal neurons, adult mice received transplants of C17.2 precursor cells (15–17) through the site of prior laser exposure as described (3). These cells express the lacZ gene encoding the β-galactosidase (β-gal) protein, detectable by an 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) histochemical reaction. They are free of helper retrovirus. A 50 nl cell suspension (1–4 × 104 cells/μl) was injected stereotaxically every 50 μm at depths from 500 μm to 250 μm from the pial surface. In some experiments (n = 11 mice), C17.2 cells were prelabeled during cell division in culture by incubation with [3H]thymidine (1 μCi/ml of medium; 1 Ci = 37 GBq) for 48 h prior to transplantation as an independent marker of donor-derived cells (3). The cage behavior of mice undergoing targeted neuronal degeneration was indistinguishable from that of intact control littermates, in agreement with prior findings (3).
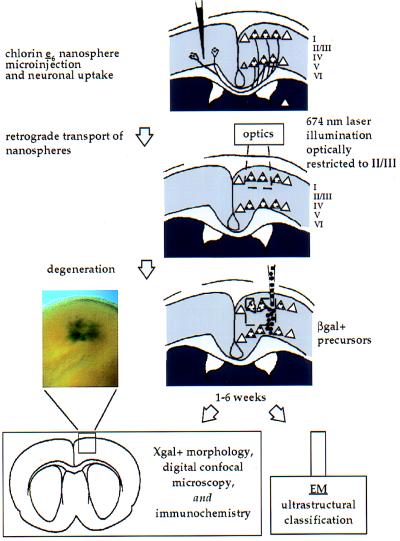
Figure 1.
Schematic of targeted neuronal degeneration and neural precursor transplantation in adult mouse neocortex. Mouse cortices were unilaterally microinjected with nanospheres carrying chlorin e6 at 5–11 days of age. Nanospheres were retrogradely transported to somata of contralateral pyramidal neurons with callosal projections. After 2–3 weeks, when mice were 3.5–5 weeks old, the contralateral cortex homotopic to the nanosphere injection site was exposed transdurally to 674 nm illumination using optics that limit penetration to layer II/III, applying uniform energy over an ≈800 μm diameter area (≈150 J/cm2). After 1–2.5 weeks, during active degeneration of targeted pyramidal neurons, adult mice received transplants of C17.2 neural precursors. Analysis was performed by bright-field, Nomarski differential interference contrast (DIC), digital confocal microscopy (DCM), electron microscopy (EM), and immunocytochemistry (ICC). Donor cells differentiated into neurons only in regions of targeted neuronal apoptosis in layer II/III [low magnification overview in Inset, corresponding to the boxed area below, shows a region ≈1 mm across in a thick section in which X-Gal+ cells can be seen variably dispersed ≈500 μm].
Control Transplants.
Two sets of control mice also received transplants of C17.2 cells. The first were intact, age-matched mice, similarly transplanted unilaterally at 6 weeks of age (n = 15). The second group consisted of age-matched mice subjected first to bilateral microinjections of low dose kainic acid (KA) within motor cortex (0.1 μl microinjections of 10 mg/ml KA administered every 50 μm from the pial surface, spanning a depth of 250 μm) at 4.5 weeks of age to produce a subtle, nonscarring lesion (3), then transplanted bilaterally 9–10 days later at 6 weeks of age (n = 15 mice; n = 30 bilateral transplants). Although such KA lesioning produced comparable cell loss 9 days after injection, it induced relatively nonspecific hypocellularity by necrosis, in contrast to specific pyramidal neuron degeneration induced by targeted neuronal apoptosis (3, 4).
Analysis of Engrafted Brains.
One to 6 weeks after transplantation, serial coronal sections of recipient brains were processed for lacZ expression by C17.2 cells using X-Gal histochemistry or anti-β-gal ICC (15–17). Sections from brains transplanted with [3H]thymidine-labeled C17.2 cells were also processed for autoradiography to identify donor cells using standard methods. Morphologic analysis was first performed at the light microscopic (LM) level, using bright-field, Nomarski DIC, and DCM (3, 15). Engrafted cells were assessed using ultrastructural criteria by EM for the direct visualization and quantitative morphometrics of cell type-specific neural components (3, 15, 19, 20). Cell type assessments were confirmed independently via ICC under LM (15). Multiple modes of analysis were performed on the same tissue. Assessments were made by two to four independent, blinded observers of random, systematically selected representative fields and EM grids from multiple serial sections spanning the transplantation region in each mouse. Sample fields ≈100–350 μm away from the injection track were evaluated at low magnification for initial assessment of transplant position and extent, then at high magnification through all focal planes using high numerical aperture optics, DIC, and DCM for morphometric analysis. Sample fields 250 μm in diameter represent ≈20% of the total area in each section into which donor cells dispersed and differentiated. Neuronal, glial, and undifferentiated phenotype assignments were made by predesignated standard criteria (listed in the section below), first quantitatively under LM as the percentage of X-Gal+ cells with neuronal phenotype for subsequent statistical analysis, then confirmed qualitatively by EM and by ICC. The ICC and EM analyses were consistent with the quantitative LM morphologic cell type assignments, but were not used for statistical analysis. Ninety percent of brains with targeted apoptotic neuronal death displayed extensive regions of well differentiated C17.2 cells, whereas 50% of intact brains and 30% of KA-lesioned brains displayed good cellular survival and differentiation of the transplanted cells.
LM.
Donor-derived cells were identified as neurons if they possessed a large soma (>15 μm diameter) and nucleus and exhibited at least two of the following structures: prominent nucleoli, axons, dendrites. Randomly selected representative fields in an average of six sections per control and experimental mouse were evaluated.
EM.
X-Gal-processed neural tissue was prepared for EM examination as described (15). The crystalline blue X-Gal reaction product is electron dense and nondiffusible, allowing donor-derived lacZ-expressing cells to be unequivocally identified and distinguished from endogenous cells (3, 15, 19, 20). X-Gal is typically found localized to the nuclear membrane, endoplasmic reticulum (ER), and other cytoplasmic organelles, and it frequently extends into cellular processes (15). A donor-derived cell was identified as a neuron under EM if it possessed at least four of six major ultrastructural criteria plus at least three of six minor ultrastructural criteria. Major criteria included (i) soma size >15 μm in diameter, (ii) large nucleus, (iii) prominent nucleolus, (iv) identifiable dendrites, (v) identifiable axon hillock, and (vi) synaptic contacts. Minor criteria included (i) oval-shaped soma, (ii) abundant heterochromatin, (iii) abundant cytoplasmic organelles (e.g., ER and mitochondria), (iv) neurofilaments (NFs) (≈10–12 nm diameter), (v) microtubules (MTs) (≈20–26 nm diameter), and (vi) myelinated processes. Between 6 and 20 systematically selected noncontiguous sections, distributed over at least two grids, were examined per experimental and control case.
ICC Under LM.
Antibodies to standard cell type-specific neural antigens were applied to sections containing lacZ+ or thymidine-labeled donor cells, in paraffin or frozen in OCT (Miles), using immunofluorescence or immunoperoxidase (Vectastain, Vector Laboratories) as described (15). Antibodies to NF (Sternberger Monoclonals), neuron-specific enolase (NSE; Calbiochem), and NeuN (21; gift from R. Mullen, University of Utah) were used as neuronal markers; an antibody to glial fibrillary acidic protein (GFAP; Boehringer Mannheim) as an astroglial marker; and antibodies to myelin 2′,3-cyclic nucleotide 3′-phosphodiesterase (CNPase; Sternberger Monoclonals) and myelin basic protein (MBP; gift of D. Colman, Mt. Sinai School of Medicine) as oligodendroglial markers. Approximately six randomly selected sections were evaluated by ICC per control and experimental animal.
Statistical Analysis.
Quantitative assignments on selected fields were repeated blindly by each observer, and compared between blinded observers, to assess single observer reproducibility and interobserver reliability. Neuronal phenotypic assignments were highly reproducible between observers and by individual observers. Interobserver variability ranged between 0% and ±18% in experimental cases, typically ±0–12%. Interobserver variability in control cases was ±0% without any neurons counted by any observer. Repeat assessments by the same observer varied by 0% to ±14%, typically ±0–3%.
Percentages of neuronal differentiation were compared using multiple statistical approaches to optimize analysis and derive the most conservative error estimates. In one set of approaches, one way ANOVA was performed to compare mean percentages of neuronal differentiation among the experimental group and the two control groups. A significant F test was followed by post hoc Student’s t tests with a Bonferroni adjustment for multiple comparisons. To maximize rigor, we analyzed outcomes using scores from either of two observers and both pooled and averaged combinations of fields. Another approach, a logistic regression model using a likelihood ratio χ2 test, considered the binary outcome for each cell (neuronal differentiation or no neuronal differentiation) and included the field/section under analysis as a categorical variable. This approach ruled out potential interactions between group and field. A two-tailed alpha level of 0.05 was considered statistically significant throughout.
RESULTS
The differentiation fate of the multipotent neural precursors (clone C17.2) was distinctly different in regions undergoing targeted apoptotic neuronal degeneration compared with the two control environments. Precursors engrafted within recipient brains of both experimental and control groups, dispersed up to 200–300 μm in all directions from the injection track. However, in intact and KA-lesioned adult neocortex, 0% differentiated into neurons; under both control conditions, the transplanted precursors either differentiated into glia (astroglia or oligodendroglia), or remained undifferentiated, by morphologic (Fig. 2), ultrastructural, and immunocytochemical (see Fig. 4) criteria. In contrast, within regions of adult neocortex undergoing targeted neuronal degeneration, 15 ± 7% (mean ± SD) of precursors present at the time of analysis had differentiated into neurons by the same criteria (see Figs. 2, 3, and 4).
Figure 2.
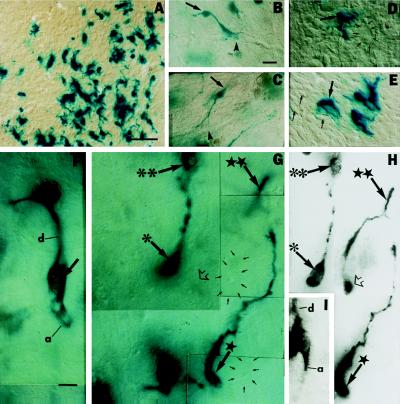
Multipotent neural precursors acquire neuronal morphology in regions of adult neocortex subjected to targeted apoptotic neuronal degeneration. They differentiate into only glia or remain undifferentiated in intact or KA-lesioned control cortex. (A) Engrafted X-Gal+ (blue) glia at low magnification 6 weeks following transplantation at 12 weeks of age; (B–E) higher magnification. (B and C) Donor-derived cells with astroglial features: small, ovoid cell bodies (arrows) with few, short processes often extending as perivascular end-feet (arrowheads). (D) Small soma of a donor-derived presumptive glial cell (arrow) compared with a much larger (≈30 μm), unlabeled, host pyramidal neuron (small arrows). (E) Donor-derived cells with oligodendroglial features (arrow): multiprocessed, ensheathing neuronal processes (short arrows). (F–I) A total of 15 ± 7% of engrafted cells in regions of neurodegeneration developed neuronal morphology, resembling pyramidal neurons within layer II/III 6 weeks following transplantation, at 12 weeks of age. (F and G) Donor-derived cells with neuronal morphology (large arrows): large somata (20–30 μm diameter) comparable to residual host pyramidal neurons (3, 4, 6) (small arrows in G outline two host neurons, visualized under DIC, each with the characteristic large nucleus and prominent nucleolus of a pyramidal neuron); 300–600 μm presumptive apical dendrites (d in F) positioned between host (small arrows) and donor (large arrows) neurons of similar morphology and size; presumptive axons (a in F). In F, the dark object at the upper end of the presumptive dendrite is another X-Gal+ cell out of the plane of focus. The donor neurons marked in G are discussed in the matching DCM image in H. (H) DCM of G, collapsing multiple planes of focus (the same symbols in both panels indicate the same cells). Cell (marked with ∗) has the characteristic large nucleus of a pyramidal neuron. Soma of cell (★★) is indicated by an open arrow in H, although all but the terminal dendrite is out of the plane of focus in G. Cells (∗∗) and neurons (★) are indicated to cross-reference between the two views of the same field in (G) and (H). (I) Two overlapping donor-derived pyramidal neurons in different focal planes show a characteristic large nucleus, prominent nucleolus, and axon (a) of the overlying cell and a prominent dendrite (d) of the underlying cell, imaged through multiple focal planes of this thick section under DCM. (Bars = 25 μm.)
Figure 4.
ICC analysis. Donor-derived cells were identified by blue X-Gal label, anti-lacZ antibody, or [3H]thymidine. Cell type-specific antibodies were visualized by fluorescent or peroxidase-conjugated secondary antibodies. The photomicrographs in A–C, D–L, and M–O are from regions of degeneration in three mice, respectively, analyzed by three distinct methods, 6 weeks following transplantation, at 12 weeks of age. (A–C) Donor-derived neurons (lacZ+ by Texas Red) immunoreactive for NeuN (fluorescein, green), a marker for mature neurons. [A and (higher magnification) B] lacZ+ cell (large arrow) double-labeled for NeuN. Other small lacZ+ cells with nonneuronal morphology were NeuN− (small arrow). Remaining host neurons (NeuN+) were lacZ− (arrowhead). (C) Donor-derived neuron (lacZ+, NeuN+; large arrow) adjacent to two NeuN− donor-derived cells (small arrow). (D–L) Donor-derived neurons identified by [3H]thymidine (silver grains) and NeuN (green). (D, G, and J) Donor-derived neurons (large arrows) with overlying silver grains, visualized by DIC. Silver grains can appear black or white under DIC, depending on lighting conditions. (E, H, and K) Same neurons under bright-field (large arrows). (F, I, and L) The same cells are NeuN+ (large arrows). A NeuN− donor-derived cell is indicated in D–F (small arrow); one of several remaining NeuN+ host neurons in J–L (arrowhead). (M–R) Immunoperoxidase (brown) and X-Gal (blue) double labeling. Overlap of the brown and blue reaction products can appear dark brown to nearly black. (M) Blue-green donor-derived cells with neuronal morphology and dark brown NF+ cell bodies (arrows) and processes (arrowheads) in regions of targeted apoptosis. These large cells (20–30 μm diameter) contrast sharply in size with those labeled by glial markers in P–R. (N and O) Morphologically identified blue donor neurons were also NSE+ (brown) (arrows), shown focused to overlap cytoplasmic NSE label with DIC imaging of the nuclei. (P) Low magnification of a control region of recipient cortex (similar to Fig. 2A) labeled by anti-MBP, an oligodendroglial marker. Among endogenous oligodendroglia (brown) are donor-derived cells (blue) also MBP+ (arrows). Glial markers labeled only small 5-to 8-μm cells. (Q) Small donor-derived cell labeled with anti-CNPase, an oligodendroglial marker (arrow). Brown CNPase+ processes extend from the blue cell (arrowheads); overlap of the X-Gal and CNPase labels appears nearly black within the soma. (R) Small blue donor-derived cell (arrow) and brown processes (arrowheads) labeled by anti-GFAP, an astroglial marker; overlap of the X-Gal and GFAP labels appears nearly black within the soma. [Bars = 25 μm (A); 10 μm (B and C); 20 μm (D, applies to E and F); 15 μm (G, applies to H and I); 10 μm (J, applies to K and L); 15 μm (M, N, and P); 10 μm (O); and 5 μm (Q and R).]
Figure 3.
Ultrastructure of donor-derived neurons in regions of adult cortex subjected to targeted apoptotic neuronal degeneration. As indicated in all panels, X-Gal precipitate (p) is visible in the nuclear membrane, cytoplasmic organelles, and processes. (A and B) EM characteristics suggestive of layer II/III pyramidal neurons are present 6 weeks following transplantation at 12 weeks of age: large somata (typically 20–30 μm); large nuclei (Nu), prominent nucleoli (n), abundant ER and mitochondria (m), and apical dendrites (d) (outlined by arrowheads). A and B show high contrast and standard contrast, respectively, of two neurons, the first chosen as lightly labeled to enhance visualization of ultrastructure, and the other more heavily labeled to best demonstrate X-Gal precipitate. An adjacent, donor-derived cellular process suggestive of an astrocytic process (a) is densely labeled with X-Gal precipitate. An afferent synapse is indicated on the donor-derived neuron in B (box, c; expanded in C). (C) Higher magnification of the axosomatic synapse boxed in B: presynaptic vesicles (white arrows) and postsynaptic specializations (large arrow); the postsynaptic region is in continuity with the donor cell nucleus via uninterrupted cytoplasm. Both the cytoplasm and the nuclear membrane contain precipitate (p). (D) Axodendritic synapse on dendrite of a donor-derived neuron: crystalline, linear X-Gal precipitate (p) in the postsynaptic region of the dendrite confirms donor origin (15, 20); postsynaptic specializations with a hazy, nonlinear, noncrystalline appearance immediately under the membrane (large arrows); presynaptic vesicles clustered near the synaptic densities (small arrows). (E) MTs (arrows, mt) of ≈20–26 nm diameter (outlined by arrowheads) in a donor-derived neuron near precipitate in the nuclear membrane (p). (F) Neurofilaments at low power in a myelinated process from a donor-derived neuron; bracket H, magnified in H, precipitate (p) is present on ≈10–12 nm diameter neurofilaments. Multiple myelin wraps on the process in (F) (bracket I), magnified in (I) (arrow), and in cross-section through a precipitate-bearing (p), donor-derived axon in G (arrowheads). (G and I) Areas where myelin separated during processing are marked (arrowheads, arrow), allowing better visualization of multiple myelin wraps. Donor-derived neurons were restricted to cortex within the degenerated region of layer II/III. [Bars = 1 μm (A and B), 100 nm (C), 200 nm (D), 40 nm (E), 500 nm (F), 200 nm (G), and 100 nm (H and I).]
This difference was highly significant and reproducible. The ANOVA established significant overall differences among the experimental and control groups (P < 0.0001; F test: F(2,45) = 36.01). Post hoc t tests indicated that the percentage of neuronal differentiation was significantly higher in the experimental group compared with the intact control group (15 vs. 0%; P < 0.0001) and compared with the KA control group (15 vs. 0%; P < 0.0001). There was no difference observed between the two control groups. There was no dependence of this P value on observer or field combination. Neuronal differentiation was also significantly greater in the experimental group compared with controls by logistical regression analysis (P < 0.0001). There was no significant interaction between group and field, indicating that the difference in percent neuronal differentiation between experimental and control groups was not dependent on the particular field observation.
Within regions of neocortex undergoing targeted apoptotic neuronal degeneration, 15 ± 7% of engrafted precursors present at the time of analysis assumed a neuronal phenotype, with many characteristics of normal pyramidal neurons within layer II/III. At the LM level, these cells displayed characteristic large somata comparable in size to endogenous pyramidal neurons, prominent axons and dendrites extending up to 300–600 μm within a single section, delicate staining of smaller axons, and intermingling with endogenous and donor neurons of similar morphology and size (Fig. 2 F–I). Donor-derived cells meeting these criteria stained positively with antibodies to NF, NSE, and NeuN (Fig. 4 E–I).
EM analysis of X-Gal+ donor cells within neocortical regions of apoptotic neuronal degeneration (Fig. 3) demonstrated defining ultrastructural characteristics of neurons, and particularly many of cortical pyramidal neurons (3, 19, 20), including size (typically 20–30 μm in diameter), morphology (large round nuclei, prominent nucleoli, abundant ER and mitochondria, large processes, apical dendrites), the presence of neuron-specific cytoskeletal elements (NFs, MTs), myelinated processes, and frequent afferent synapse formation. In 27 representative fields, 79 donor-derived processes were myelinated and contained NFs; 32 contained MTs. NFs had a mean diameter of 11 ± 1.4 nm (n = 177 individual NFs in 17 representative donor-derived neuronal processes in 10 separate fields). MTs had a mean diameter of 24 ± 1.7 nm (n = 68 individual MTs in 10 donor-derived neurons in 10 separate fields). Myelin averaged 5 ± 2 wraps per process. Many transplant-derived neurons received axosomatic and axodendritic synapses. Eighty-four synapses (43% axosomatic) were observed on 20 such donor-derived neurons sampled from 27 fields in these single, nonserial sections, suggesting relatively high synaptic density.
In contrast, donor-derived cells within control mice contained cells lacking these ultrastructural, immunocytochemical, and morphologic characteristics. Morphologically, the majority of donor-derived cells in control mice possessed small (5–8 μm diameter), ovoid cell bodies with a few, short processes often extending as perivascular foot processes onto cerebral blood vessels, features suggestive of astroglia (Fig. 2 B and C). A smaller percentage of these donor-derived glia were multiprocessed and ensheathed neuronal processes, characteristics suggestive of an oligodendroglial phenotype (Fig. 2 D and E). Both types of presumptive glia displayed the appropriate defining ultrastructural profiles (e.g., intermediate glial filaments for astroglia; dark, granular cytoplasm and nucleus for oligodendroglia). In all recipient animals, many donor cells persisted in a less differentiated state, characterized by (i) somata which were 5–10 μm in diameter, (ii) relative lack of Nissl substance, (iii) scalloped nuclei lacking a nucleolus, and (iv) absence of intermediate filaments, MTs, or dense granules. Morphologically identified presumptive glia stained positively with antibodies appropriate to their respective cell type (e.g., GFAP for astroglia, and MBP and CNPase for oligodendroglia) (Fig. 4). Glial differentiation in the controls is consistent with the fact that gliogenesis is the predominant differentiative process in postnatal neocortex.
Neuronal differentiation was limited to the regions of targeted neuronal apoptosis in lamina II/III. Engrafted donor cells in immediately deeper layers of neocortex (IV, V, and VI) and corpus callosum differentiated into glia or remained undifferentiated, like those in the two control groups.
DISCUSSION
Approximately 15% of multipotent neural precursors present at the time of analysis had differentiated into neurons in regions of adult mouse neocortex undergoing apoptotic degeneration of targeted pyramidal neurons. Cortical neuronal differentiation of these cells otherwise occurs only during embryonic corticogenesis (16) and not in intact or nonspecifically lesioned adult neocortex. The directed differentiation of neural precursors within regions of apoptotic neuronal death in adult neocortex suggests that this form of degeneration created a microenvironment permissive and/or instructive for neuronal differentiation of these clonal, multipotent precursors through reexpression of developmental signals ordinarily available only during embryonic corticogenesis. This hypothesis is supported by recent findings of specific, temporally distinct up-regulation of the neurotrophins BDNF, NT-4/5, and NT-3 by interneurons that are synaptic neighbors of targeted degenerating neurons while they undergo apoptosis, suggesting specific intercellular signaling (22). It is likely that these factors represent a subset of a larger set of molecules not yet identified.
We favor the interpretation that these or other signals have actively influenced the differentiation of these multipotent precursors, for a number of reasons. It is not the case that transplanted precursors simply failed to engraft or died in intact and KA-lesioned brains; C17.2 cells engrafted and survived in both experimental and control conditions. Within the proper developmental context, following implantation into the fetal ventricular zone, C17.2 precursors have the ability to differentiate into both pyramidal neurons and glia (16); but in regions where neurogenesis has ceased and gliogenesis persists, they differentiate only as glia (16). This dependence on developmental context reinforces the concept that microenvironmental signals guide their developmental fate.
Under conditions of ongoing apoptotic degeneration, it appears that these precursors are capable of undergoing directed neuronal differentiation and of partially repopulating a region of degenerating neurons. Donor-derived neurons possessed many of the morphologic, ultrastructural, and immunocytochemical characteristics of layer II/III adult pyramidal neurons. They received synaptic contacts, they contained appropriate cytoskeletal elements, and their axons and dendrites became myelinated.
Many CNS injury models, including excitotoxic, hypoxic, or surgical lesions, are complicated by multiple uncontrolled pathologic processes affecting multiple cell types. Similarly, the interpretation of experiments using mutant animal strains is often confounded by a poor understanding of the etiology underlying neurodegeneration. Targeted apoptotic neurodegeneration is well characterized and well controlled. The relatively synchronous onset of this experimentally induced neuronal apoptosis may allow a concentration of up-regulated signals to guide differentiation in a manner difficult to discern in sporadic CNS degeneration.
Our experiments provide evidence that significant plasticity resides at the level of the individual precursor. Though fetal neural tissue has been grafted into adult rodent brain (1), and transplanted primary embryonic cortical neurons respond to developmental signals altered by targeted cell death (3, 4, 6), fetal tissue poses significant problems of availability of sufficient, well-characterized donor tissue, and a lack of homogeneity of the cellular population under study. Clonal neural precursor lines are plentiful, homogeneous, and highly characterized. Our data support the hypothesis that clonal, multipotent neural precursors can respond differently to modified microenvironmental signals and undergo directed neuronal differentiation even in the adult CNS (3, 4, 8). The findings also support the hypothesis that induced neuronal apoptosis is a good model to study the molecular signals that govern neuronal differentiation.
The use of a defined precursor population within a systematically perturbed local environment may facilitate the future isolation of factors and genes pivotal in directing neuronal commitment and differentiation in normal and abnormal mammalian CNS. The robust lacZ expression suggests that foreign genes of therapeutic or developmental importance may also be expressed by transplanted precursors within regions of neurodegeneration (17, 23, 24). Our data suggest a paradigm of neural precursor transplantation as a possible cell replacement or molecular support therapy for some degenerative, developmental, and acquired diseases of neocortex and other CNS structures.
Acknowledgments
We thank V. Sheen and B. Leavitt for scientific input; C. Austin, L. Benowitz, S. Pomeroy, and S. Whittemore for comments on the manuscript; and D. Zurakowski for statistical advice. This work was supported by grants from the National Institutes of Health (HD28478, NS33852) and Alzheimer’s Association (IIRG-93-143, RG1-96-045) to J.D.M.; from the National Institutes of Health (NS33852, NS34247), the American Paralysis Association, and the Paralyzed Veterans of America to E.Y.S.; and Mental Retardation Research Center Grant HD18655. C.Y. and J.D.F. were trainees on Grant NS07264. J.D.M. was a Rita Allen Foundation Scholar.
ABBREVIATIONS
- CNS
central nervous system
- EM
electron microscopy
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
- KA
kainic acid
- ICC
immunocytochemistry
- LM
light microscopic
- DIC
differential interference contrast
- ER
endoplasmic reticulum
- NF
neurofilament
- MT
microtubule
- NSE
neuron-specific enolase
- GFAP
glial fibrillary acidic protein
- CNPase
2′,3-cyclic nucleotide 3′-phosphodiesterase
- MBP
myelin basic protein
- β-gal
β-galactosidase
References
- 1.Fisher L J, Gage F H. Physiol Rev. 1993;73:583–616. doi: 10.1152/physrev.1993.73.3.583. [DOI] [PubMed] [Google Scholar]
- 2.Macklis J D, Madison R D. J Neurosci. 1991;11:2055–2062. doi: 10.1523/JNEUROSCI.11-07-02055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macklis J D. J Neurosci. 1993;13:3848–3863. doi: 10.1523/JNEUROSCI.13-09-03848.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheen V L, Macklis J D. J Neurosci. 1995;15:8378–8392. doi: 10.1523/JNEUROSCI.15-12-08378.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheen V L, Macklis J D. Exp Neurol. 1994;130:67–81. doi: 10.1006/exnr.1994.1186. [DOI] [PubMed] [Google Scholar]
- 6.Hernit-Grant C S, Macklis J D. Exp Neurol. 1996;139:131–142. doi: 10.1006/exnr.1996.0088. [DOI] [PubMed] [Google Scholar]
- 7.Bredesen D E. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- 8.Whittemore S R, Snyder E Y. Mol Neurobiol. 1996;12:13–38. doi: 10.1007/BF02740745. [DOI] [PubMed] [Google Scholar]
- 9.Lois C, Alvarez-Buylla A. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 10.Campbell K, Olsson M, Bjorklund A. Neuron. 1995;15:1259–1273. doi: 10.1016/0896-6273(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 11.Brustle O, Maskos U, McKay R D G. Neuron. 1995;15:1275–1285. doi: 10.1016/0896-6273(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 12.Suhonen J O, Peterson D A, Ray J, Gage F H. Nature (London) 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- 13.Weiss S, Reynolds B A, Vescovi A L, Morshead C, Craig C, van der Kooy D. Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick T, Richards L J, Bartlett P F. Mol Cell Neurosci. 1995;6:2–15. doi: 10.1006/mcne.1995.1002. [DOI] [PubMed] [Google Scholar]
- 15.Snyder E Y, Deitcher D L, Walsh C, Arnold-Aldea S, Hartwieg E A, Cepko C L. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 16.Snyder E Y, Yandava B D, Pan Z-H, Yoon C H, Macklis J D. Soc Neurosci Abstr. 1993;19:613. [Google Scholar]
- 17.Snyder E Y, Taylor R M, Wolfe J H. Nature (London) 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 18.Gage F H, Ray J, Fisher L J. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 19.Peters A, Palay S L, Webster H D. The Fine Structure of the Nervous System, Neurons and Their Supporting Cells. Oxford: Oxford Univ. Press; 1991. [Google Scholar]
- 20.Luskin M B, Parnavelas J G, Barfield J A. J Neurosci. 1993;13:1730–1750. doi: 10.1523/JNEUROSCI.13-04-01730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullen R J, Buck C R, Smith A M. Development (Cambridge, UK) 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Sheen V L, Macklis J D. Soc Neurosci Abstr. 1996;22:1210. [Google Scholar]
- 23.Martinez-Serrano A, Bjorklund A. Clin Neurosci. 1996;3:301–309. [PubMed] [Google Scholar]
- 24.Lacorazza H D, Flat J D, Snyder E Y, Jendoubi M. Nat Med. 1996;4:424–429. doi: 10.1038/nm0496-424. [DOI] [PubMed] [Google Scholar]