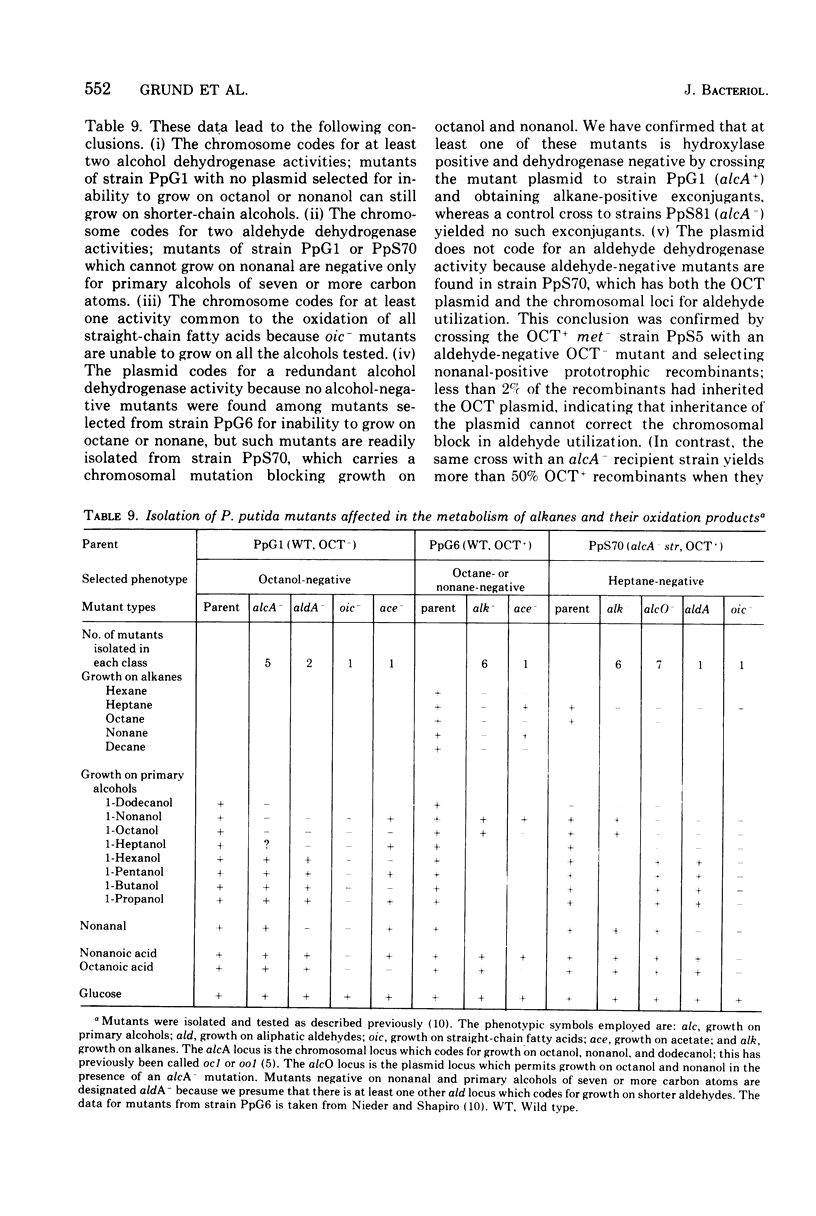
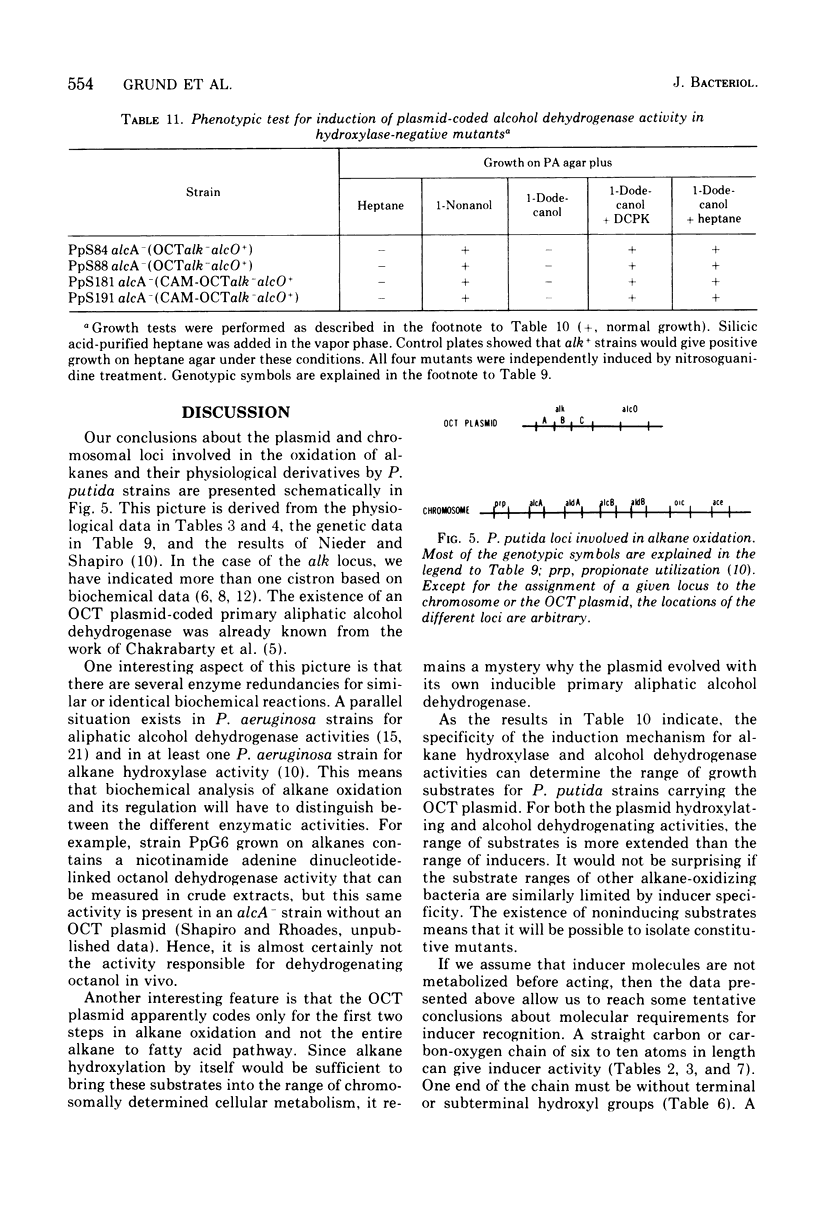
Abstract
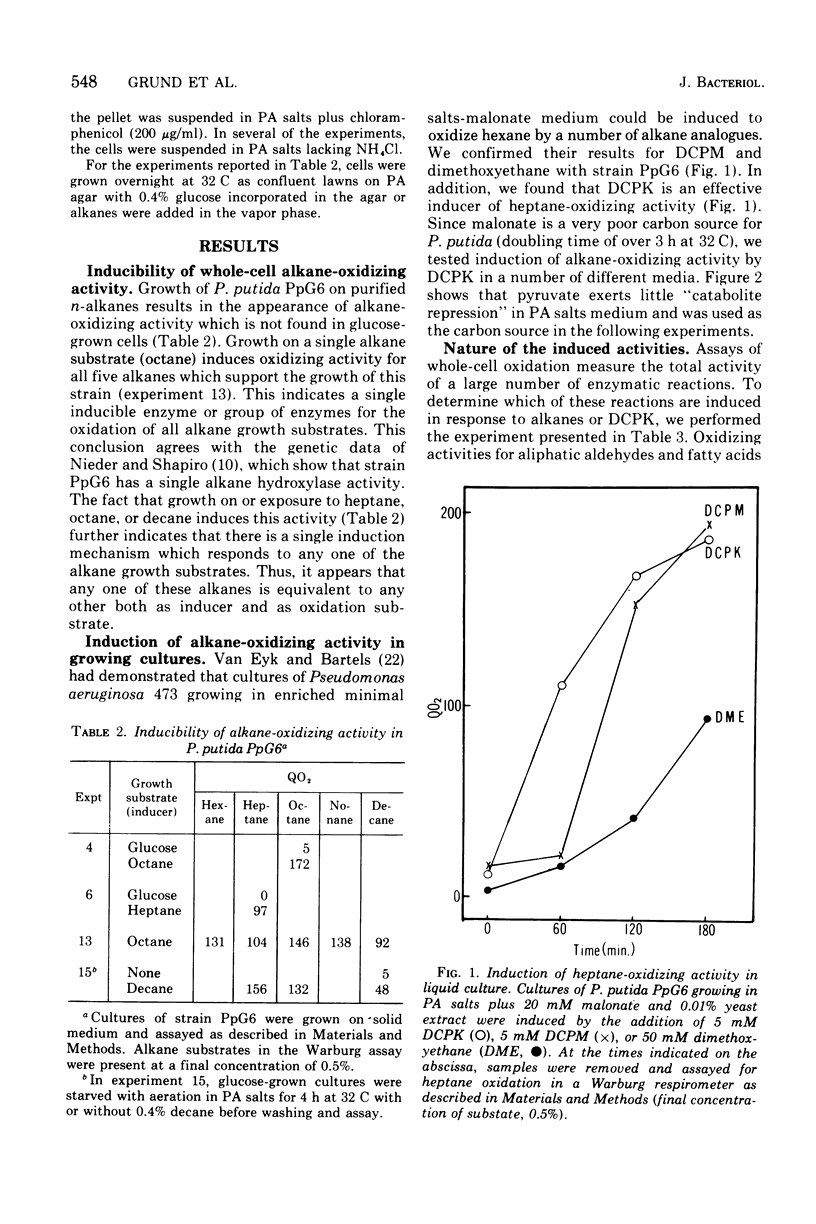
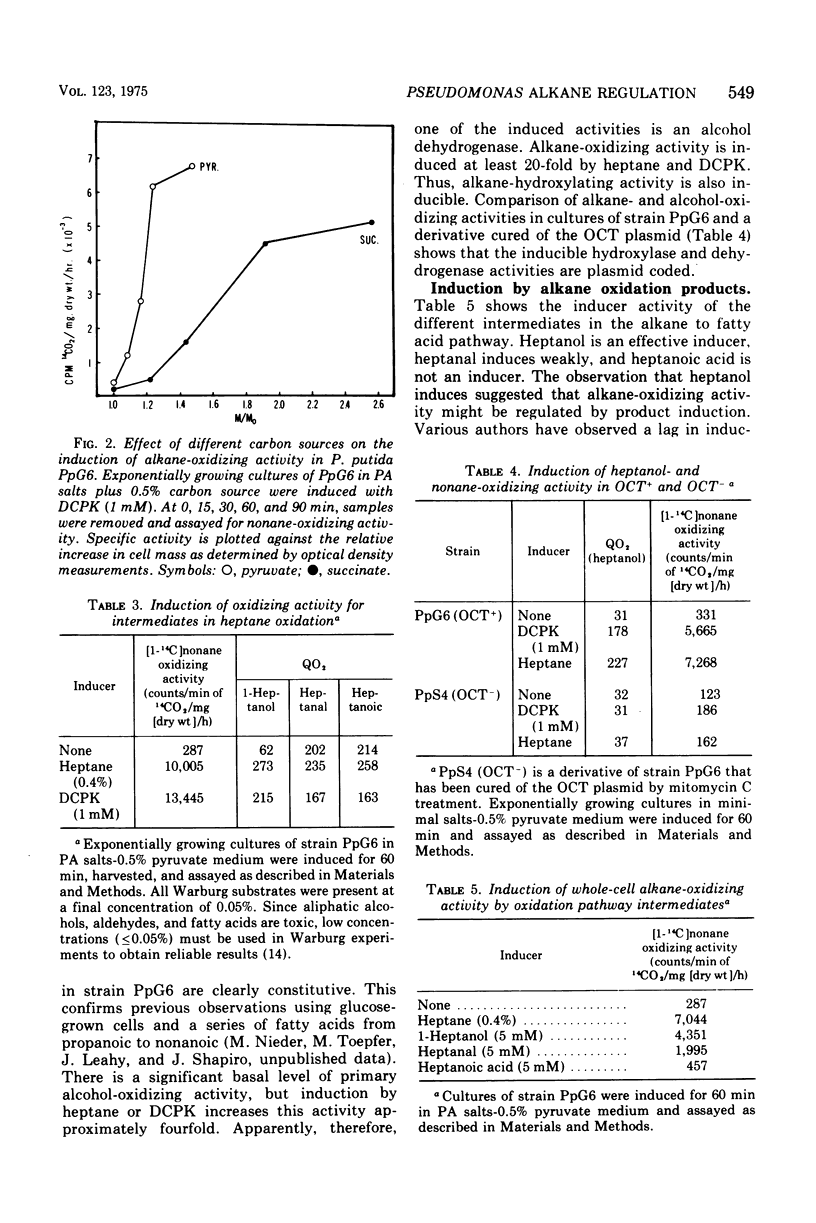
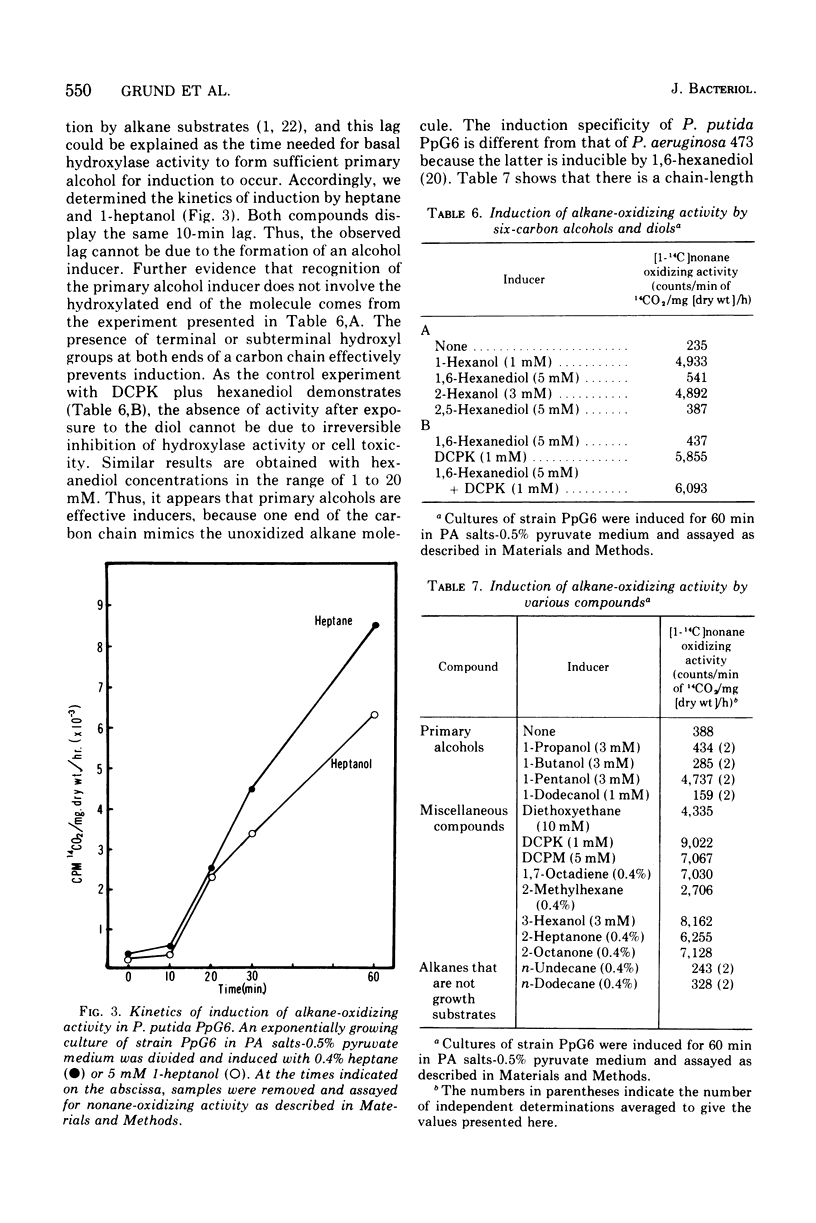
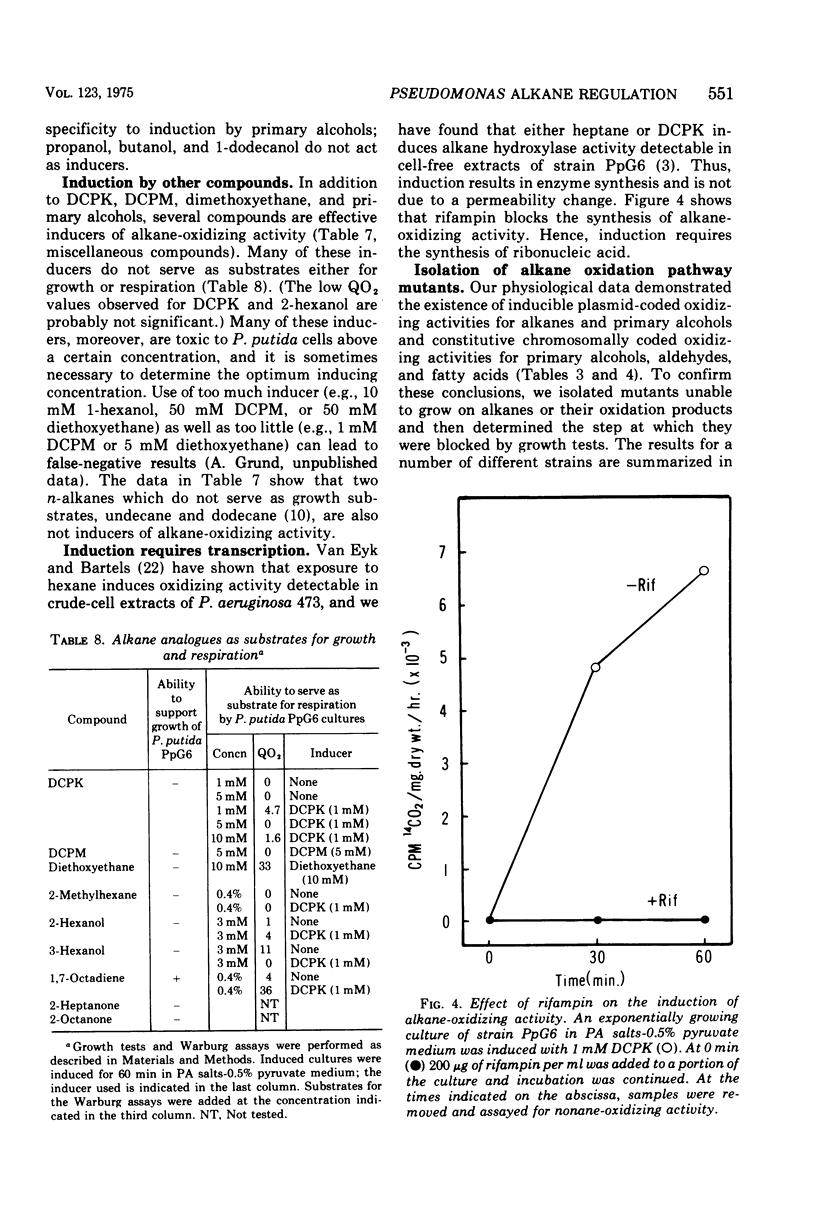
We have studied the appearance of whole-cell oxidizing activity for n-alkanes and their oxidation products in strains of Pseudomonas putida carrying the OCT plasmid. Our results indicate that the OCT plasmid codes for inducible alkane-hydroxylating and primary alcohol-dehydrogenating activities and that the chromosome codes for constitutive oxidizing activities for primary alcohols, aliphatic aldehydes, and fatty acids. Mutant isolation confirms the presence of an alcohol dehydrogenase locus on the OCT plasmid and indicated the presence of multiple alcohol and aldehyde dehydrogenase loci on the P. putida chromosome. Induction tests with various compounds indicate that inducer recognition has specificity for chain length and can be affected by the degree of oxidation of the carbon chain. Some inducers are neither growth nor respiration substrates. Growth tests with and without a gratuitous inducer indicate that undecane is not a growth substrate because it does not induce alkane hydroxylase activity. Using a growth test for determining induction of the plasmid alcohol dehydrogenase it is possible to show that heptane induces this activity in hydroxylase-negative mutants. This suggests that unoxidized alkane molecules are the physiological inducers of both plasmid activities.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZOULAY E., SENEZ J. C. [Bacterial degradation of paraffin hydrocarbons. II. Determination of intermediary products by the simultaneous adaptation method]. Ann Inst Pasteur (Paris) 1960 Jun;98:868–879. [PubMed] [Google Scholar]
- BAPTIST J. N., GHOLSON R. K., COON M. J. Hydrocarbon oxidation by a bacterial enzyme system. I. Products of octane oxidation. Biochim Biophys Acta. 1963 Jan 1;69:40–47. doi: 10.1016/0006-3002(63)91223-x. [DOI] [PubMed] [Google Scholar]
- Benson S., Shapiro J. Induction of alkane hydroxylase proteins by unoxidized alkane in Pseudomonas putida. J Bacteriol. 1975 Aug;123(2):759–760. doi: 10.1128/jb.123.2.759-760.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Chou G., Gunsalus I. C. Genetic regulation of octane dissimilation plasmid in Pseudomonas. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1137–1140. doi: 10.1073/pnas.70.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Genetic fusion of incompatible plasmids in Pseudomonas. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1641–1644. doi: 10.1073/pnas.70.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHOLSON R. K., BAPTIST J. N., COON M. J. HYDROCARBON OXIDATION BY A BACTERIAL ENZYME SYSTEM. II. COFACTOR REQUIREMENTS FOR OCTANOL FORMATION FROM OCTANE. Biochemistry. 1963 Sep-Oct;2:1155–1159. doi: 10.1021/bi00905a043. [DOI] [PubMed] [Google Scholar]
- HERINGA J. W., HUYBREGTSE R., van der LINDEN A. n-Alkane oxidation by a Pseudomonas. Formation and beta-oxidation of intermediate fatty acids. Antonie Van Leeuwenhoek. 1961;27:51–58. doi: 10.1007/BF02538422. [DOI] [PubMed] [Google Scholar]
- KUSUNOSE M., KUSUNOSE E., COON M. J. ENZYMATIC OMEGA-OXIDATION OF FATTY ACIDS. II. SUBSTRATE SPECIFICITY AND OTHER PROPERTIES OF THE ENZYME SYSTEM. J Biol Chem. 1964 Jul;239:2135–2139. [PubMed] [Google Scholar]
- McKenna E. J., Coon M. J. Enzymatic omega-oxidation. IV. Purification and properties of the omega-hydroxylase of Pseudomonas oleovorans. J Biol Chem. 1970 Aug 10;245(15):3882–3889. [PubMed] [Google Scholar]
- Nieder M., Shapiro J. Physiological function of the Pseudomonas putida PpG6 (Pseudomonas oleovorans) alkane hydroxylase: monoterminal oxidation of alkanes and fatty acids. J Bacteriol. 1975 Apr;122(1):93–98. doi: 10.1128/jb.122.1.93-98.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. J., Scheld H. W. Oxidation of hydrocarbons by microorganisms isolated from soil. Can J Microbiol. 1968 Apr;14(4):403–407. doi: 10.1139/m68-064. [DOI] [PubMed] [Google Scholar]
- Peterson J. A., Kusunose M., Kusunose E., Coon M. J. Enzymatic omega-oxidation. II. Function of rubredoxin as the electron carrier in omega-hydroxylation. J Biol Chem. 1967 Oct 10;242(19):4334–4340. [PubMed] [Google Scholar]
- ROBINSON D. S. OXIDATION OF SELECTED ALKANES AND RELATED COMPOUNDS BY A PSEUDOMONAS STRAIN. Antonie Van Leeuwenhoek. 1964;30:303–316. doi: 10.1007/BF02046736. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Chakrabarty A. M., Gunsalus I. C. A transmissible plasmid controlling camphor oxidation in Pseudomonas putida. Proc Natl Acad Sci U S A. 1973 Mar;70(3):885–889. doi: 10.1073/pnas.70.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIJSSE G. J., van der LINDEN A. Pathways of hydrocarbon dissimilation by a Pseudomonas as revealed by chloramphenicol. Antonie Van Leeuwenhoek. 1963;29:89–100. doi: 10.1007/BF02046042. [DOI] [PubMed] [Google Scholar]
- Van der Linden A. C., Huybregtse R. Occurrence of inducible and NAD(P)-independent primary alcohol dehydrogenases in an alkane-oxidizing Pseudomonas. Antonie Van Leeuwenhoek. 1969;35(3):344–360. doi: 10.1007/BF02219154. [DOI] [PubMed] [Google Scholar]
- van Eyk J., Bartels T. J. Paraffin oxidation in Pseudomonas aeruginosa. I. Induction of paraffin oxidation. J Bacteriol. 1968 Sep;96(3):706–712. doi: 10.1128/jb.96.3.706-712.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


