Abstract
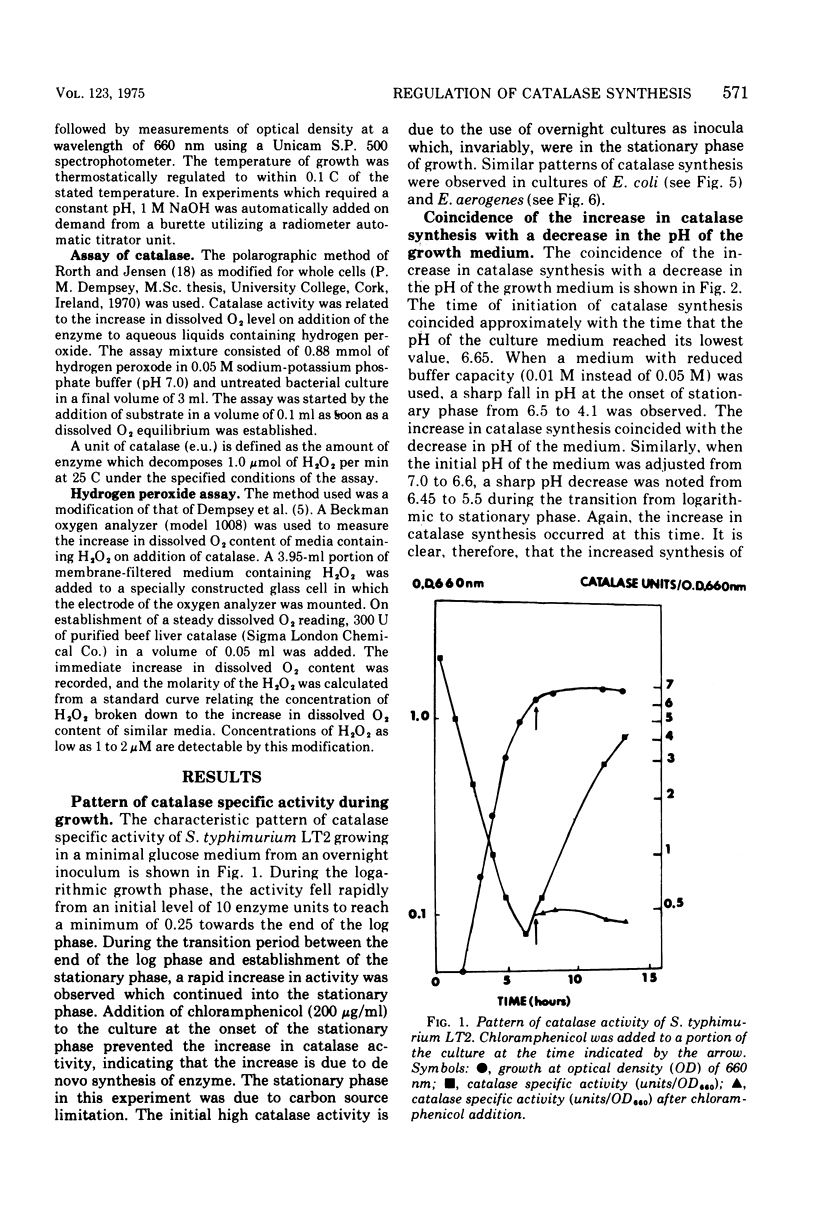
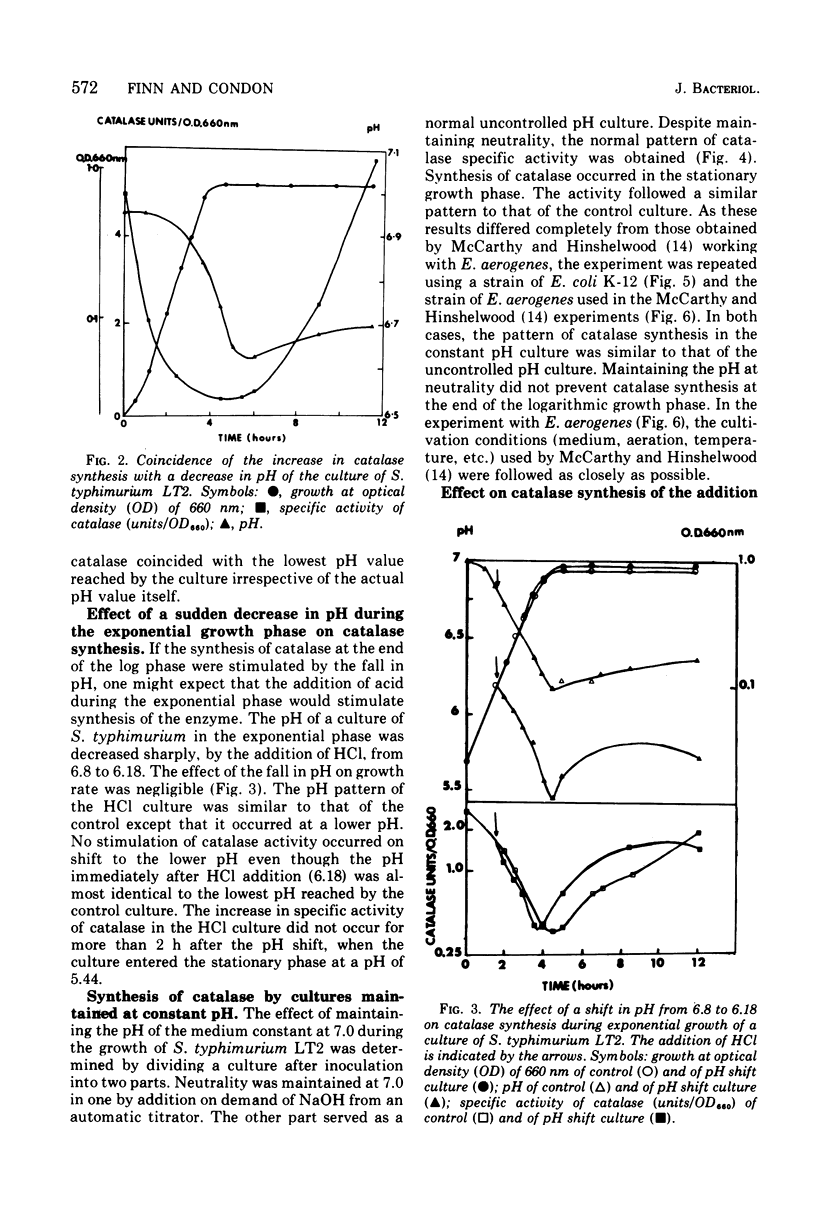
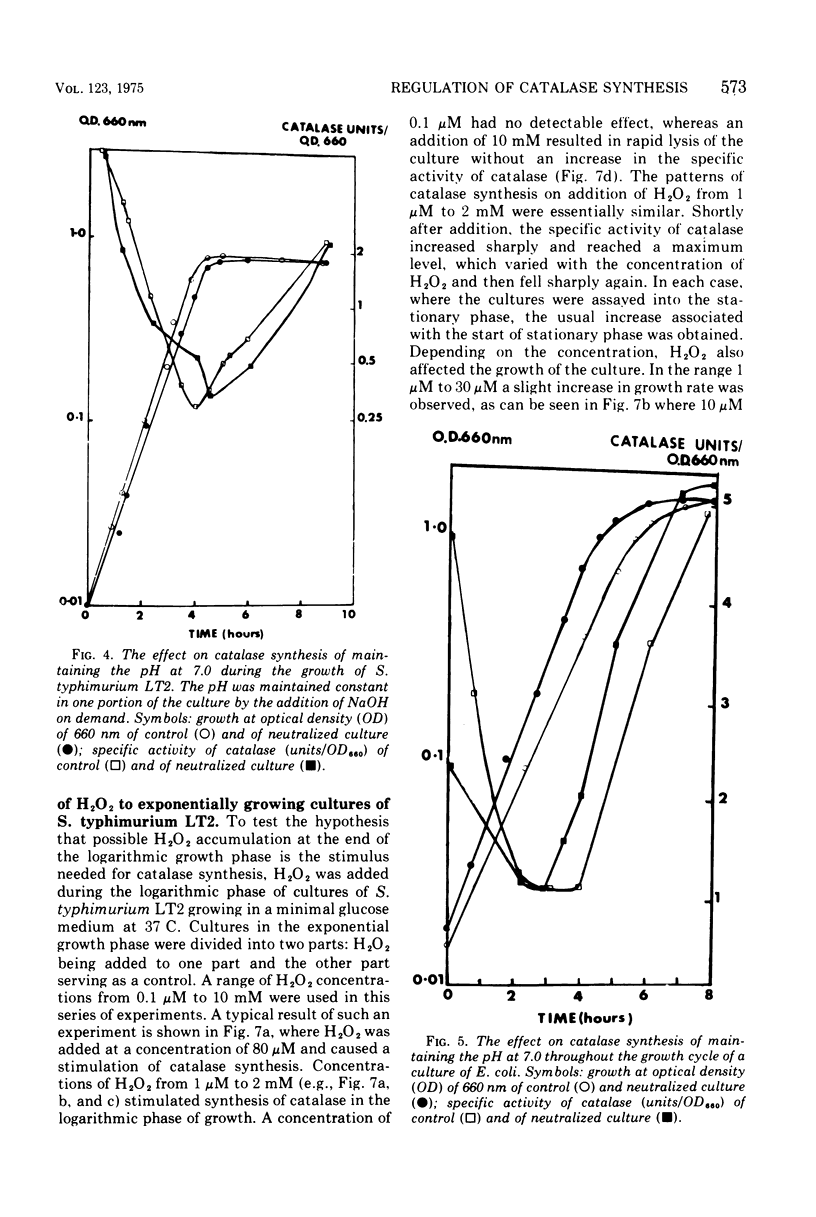
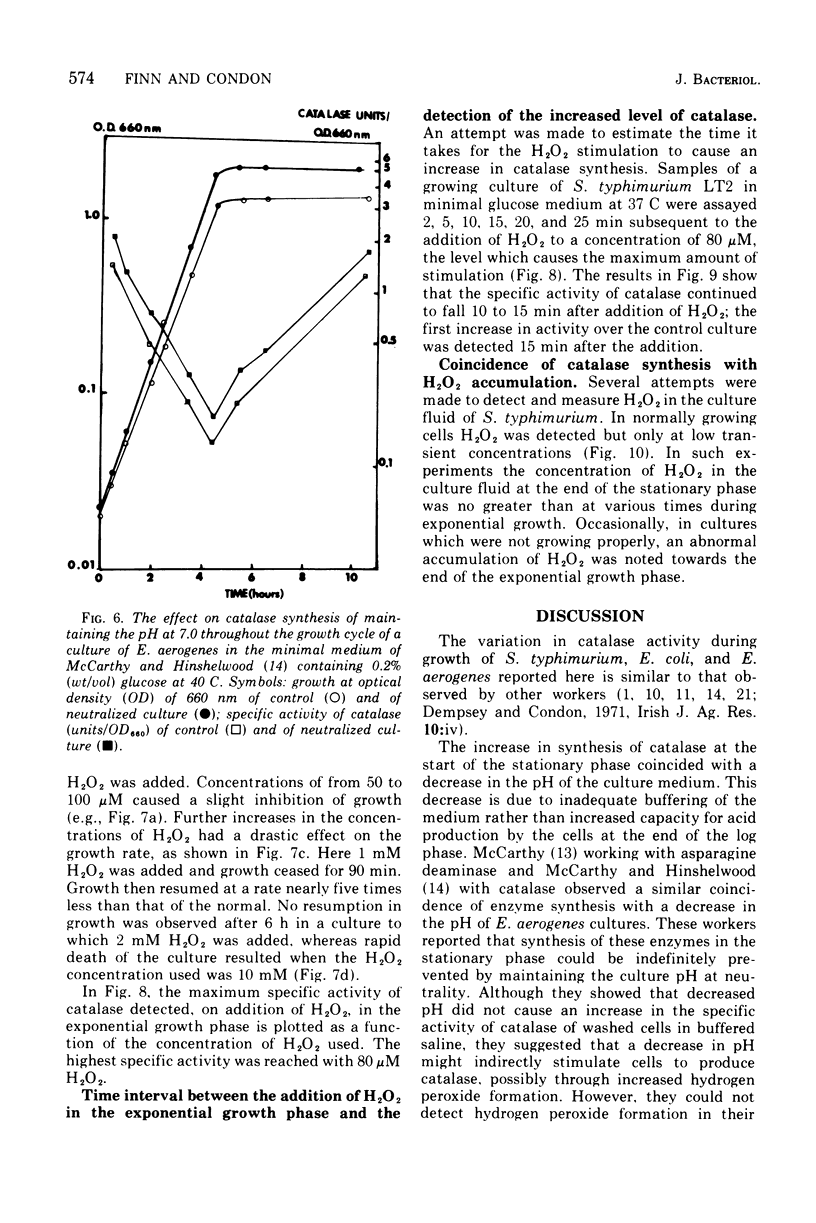
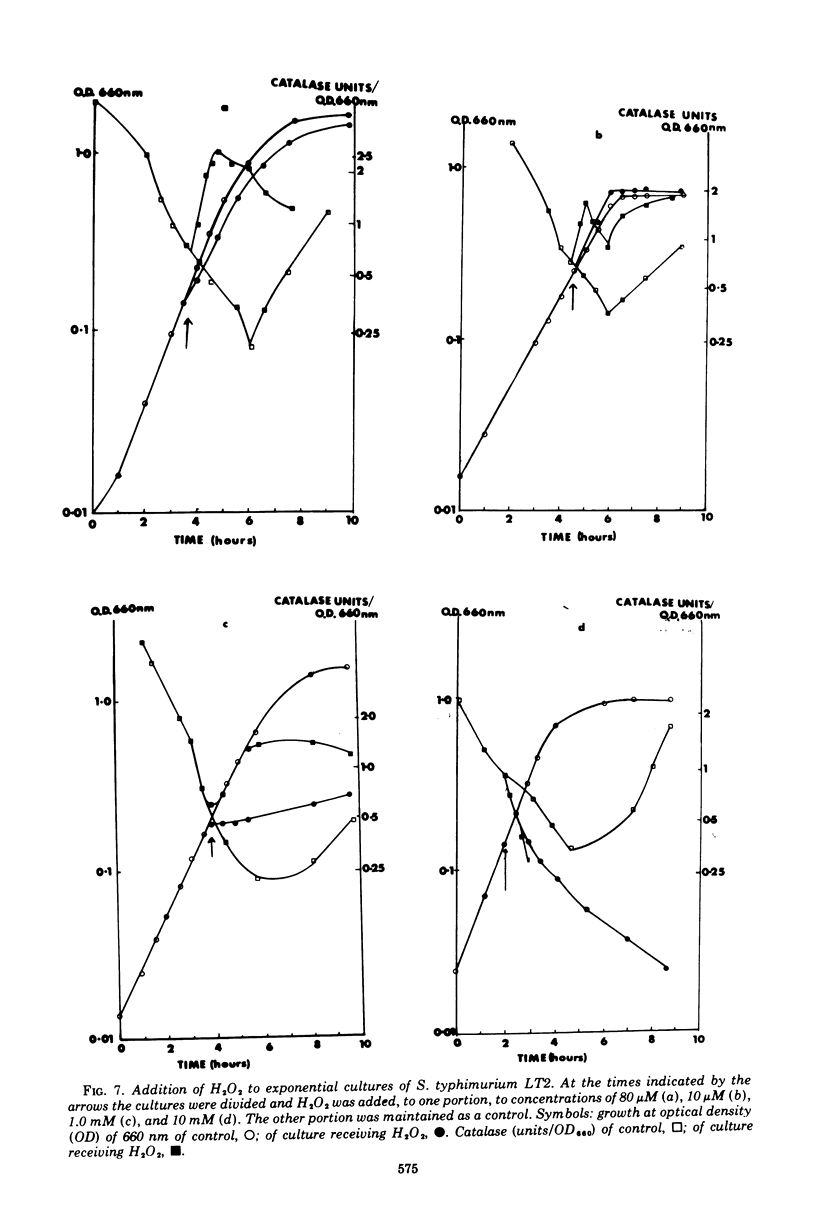
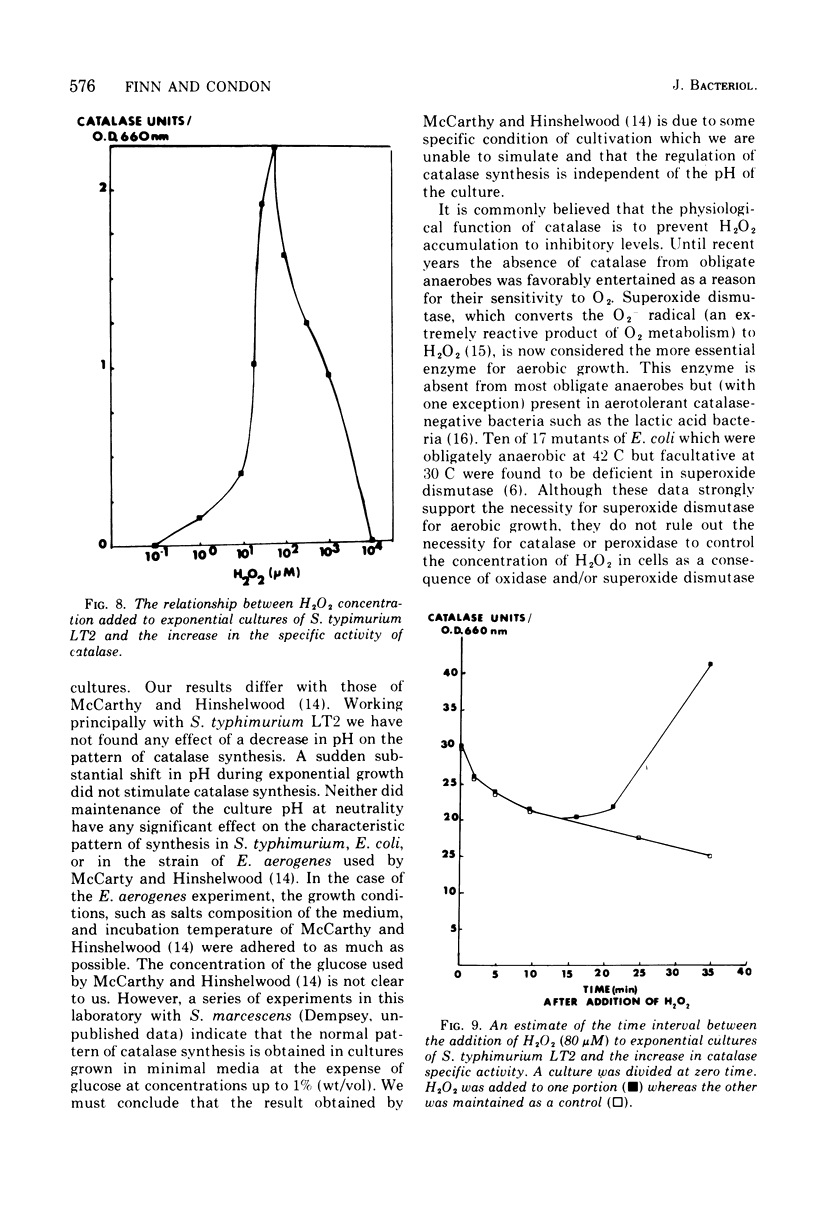
The specific activity of catalase in Salmonella typhimurium and other enteric bacteria decreased during the logarithmic phase of growth and increased at the onset and during the stationary phase. The increase in catalase synthesis at the end of the exponential phase in S. typhimurium cells coincided with the lowest pH value reached by the culture. Maintenance of the pH at a constant neutral value did not alter the typical pattern of synthesis in contradiction of the results previously reported (McCarthy and Hinshelwood. 1959). A sudden decrease in the pH value of an S. typhimurium culture during exponential growth by addition of HC1 did not cause an alteration in the catalase synthesis pattern. Addition of hydrogen peroxide to S. typhimurium cultures within the range 1 muM TO 2MM during the exponential growth phase stimulated catalase synthesis. The extent of catalase synthesis depended on the concentration of hydrogen peroxide; the maximum stimulation was observed at 80 muM. Increased catalase synthesis was not detected for 10 to 15 min after hydrogen peroxide addition. Hydrogen peroxide was produced by S. typhimurium cultures during the exponential and stationary growth phases. However, no direct relationship between hydrogen peroxide accumulation and synthesis of catalase was observed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Hogg D. M., Jago G. R. Formation of hydrogen peroxide by group N streptococci and its effect on their growth and metabolism. Appl Microbiol. 1970 Apr;19(4):608–612. doi: 10.1128/am.19.4.608-612.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. An intermediate stage in the hydrogen peroxide-induced synthesis of catalase in Rhodopseudomonas spheroides. J Cell Comp Physiol. 1960 Feb;55:9–14. doi: 10.1002/jcp.1030550103. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. Physiology of induced catalase synthesis in Rhodopseudomonas spheroides. J Cell Comp Physiol. 1960 Feb;55:1–7. doi: 10.1002/jcp.1030550102. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. The induced synthesis of catalase in Rhodopseudomonas spheroides. Biochim Biophys Acta. 1960 Jan 29;37:503–512. doi: 10.1016/0006-3002(60)90507-2. [DOI] [PubMed] [Google Scholar]
- Dempsey P. M., O'Leary J., Condon S. Polarographic assay of hydrogen peroxide accumulation in microbial cultures. Appl Microbiol. 1975 Feb;29(2):170–174. doi: 10.1128/am.29.2.170-174.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY E. D., HAYWOOD A. M., CHARGAFF E. RAPIDLY LABELED RIBONUCLEIC ACIDS OF RHODOPSEUDOMONAS SPHEROIDES UNDER VARYING CONDITIONS OF CATALASE SYNTHESIS. Biochim Biophys Acta. 1964 Jul 22;87:397–415. doi: 10.1016/0926-6550(64)90113-6. [DOI] [PubMed] [Google Scholar]
- KOVACS E., MAZAREAN H. H., JAKI A. UNTERSUCHUNGEN UEBER DEN ZUSAMMENHANG ZWISCHEN KATALASEYSNTHESE UND ATMUNGSINTENSITAET IN STAPHYLOCOCCUS AUREUS-KULTUREN. Enzymologia. 1965 May 15;28:316–321. [PubMed] [Google Scholar]
- Kepes A., Beguin S. Peptide chain initiation and growth in the induced synthesis of beta-galactosidase. Biochim Biophys Acta. 1966 Sep;123(3):546–560. doi: 10.1016/0005-2787(66)90222-x. [DOI] [PubMed] [Google Scholar]
- Kwiek S., Gabryś A., Witecki J. The effect of glucose and galactose on catalase activity of Salmonella typhimurium in aerobic and anaerobic cultures. Acta Microbiol Pol B. 1970;2(2):115–120. [PubMed] [Google Scholar]
- Lacroute F., Stent G. S. Peptide chain growth of -galactosidase in Escherichia coli. J Mol Biol. 1968 Jul 14;35(1):165–173. doi: 10.1016/s0022-2836(68)80044-0. [DOI] [PubMed] [Google Scholar]
- Mazarean H. H., Kovács E. The effect of thiol-compounds on catalase activity. Enzymologia. 1966 Sep 30;31(3):184–188. [PubMed] [Google Scholar]
- McCARTHY B. J., HINSHELWOOD C. Variations in catalase activity during a bacterial growth cycle. Proc R Soc Lond B Biol Sci. 1959 Jan 27;150(938):13–23. doi: 10.1098/rspb.1959.0004. [DOI] [PubMed] [Google Scholar]
- McCARTHY B. J. Variations in dehydrogenase and deaminase activity during bacterial growth. Proc R Soc Lond B Biol Sci. 1959 Apr 21;150(940):410–422. doi: 10.1098/rspb.1959.0031. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth M., Jensen P. K. Determination of catalase activity by means of the Clark oxygen electrode. Biochim Biophys Acta. 1967 May 16;139(1):171–173. doi: 10.1016/0005-2744(67)90124-6. [DOI] [PubMed] [Google Scholar]
- Shanmugam K. T., Berger L. R. Mechanism of catalase induction in Rhodopseudomonas spheroides: role of porphyrin excretion. Arch Mikrobiol. 1969;69(3):197–205. doi: 10.1007/BF00408972. [DOI] [PubMed] [Google Scholar]
- Sulebele G. A., Rege D. V. The nature of the glucose effect on the induced synthesis of catalase in Saccharomyces cerevisiae. Enzymologia. 1968 Dec 31;35(6):321–334. [PubMed] [Google Scholar]
- WHITE D. C. Cytochrome and catalase patterns during growth of Haemophilus parainfluenzae. J Bacteriol. 1962 Apr;83:851–859. doi: 10.1128/jb.83.4.851-859.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]


