Abstract
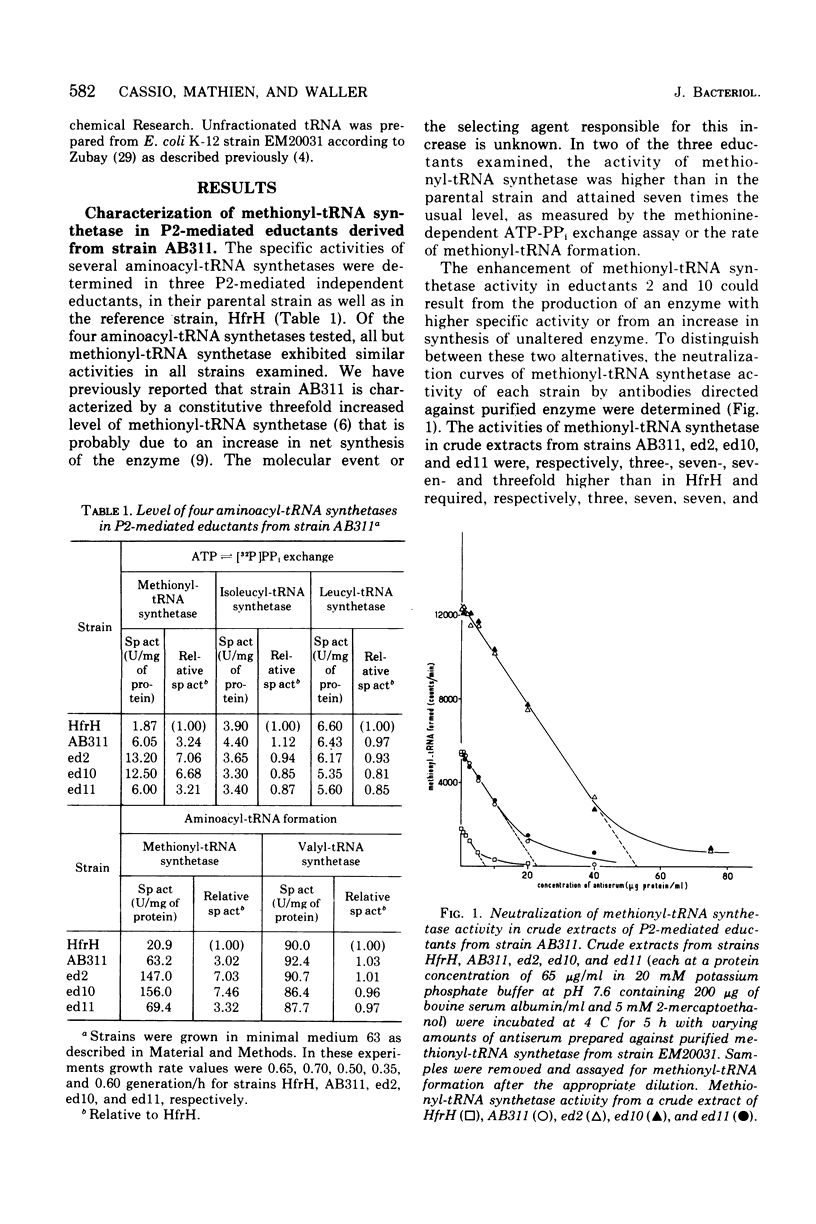
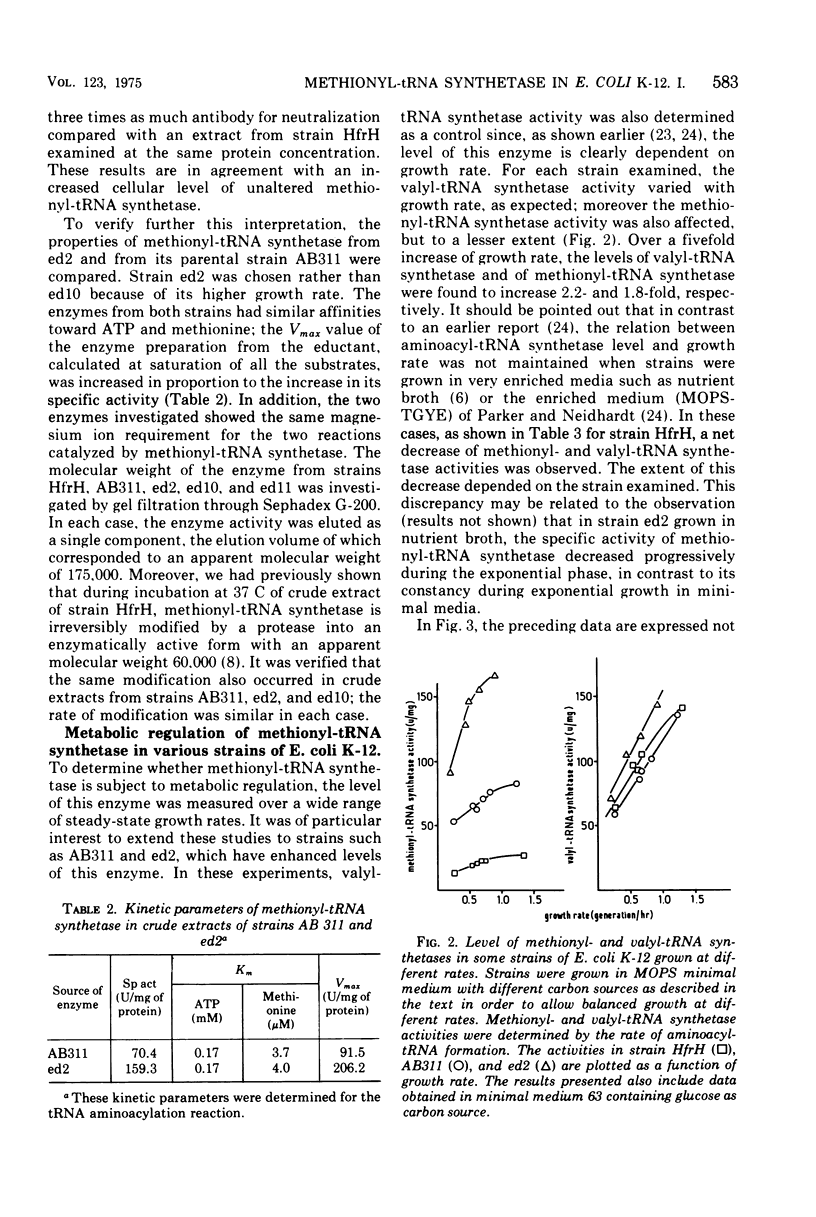
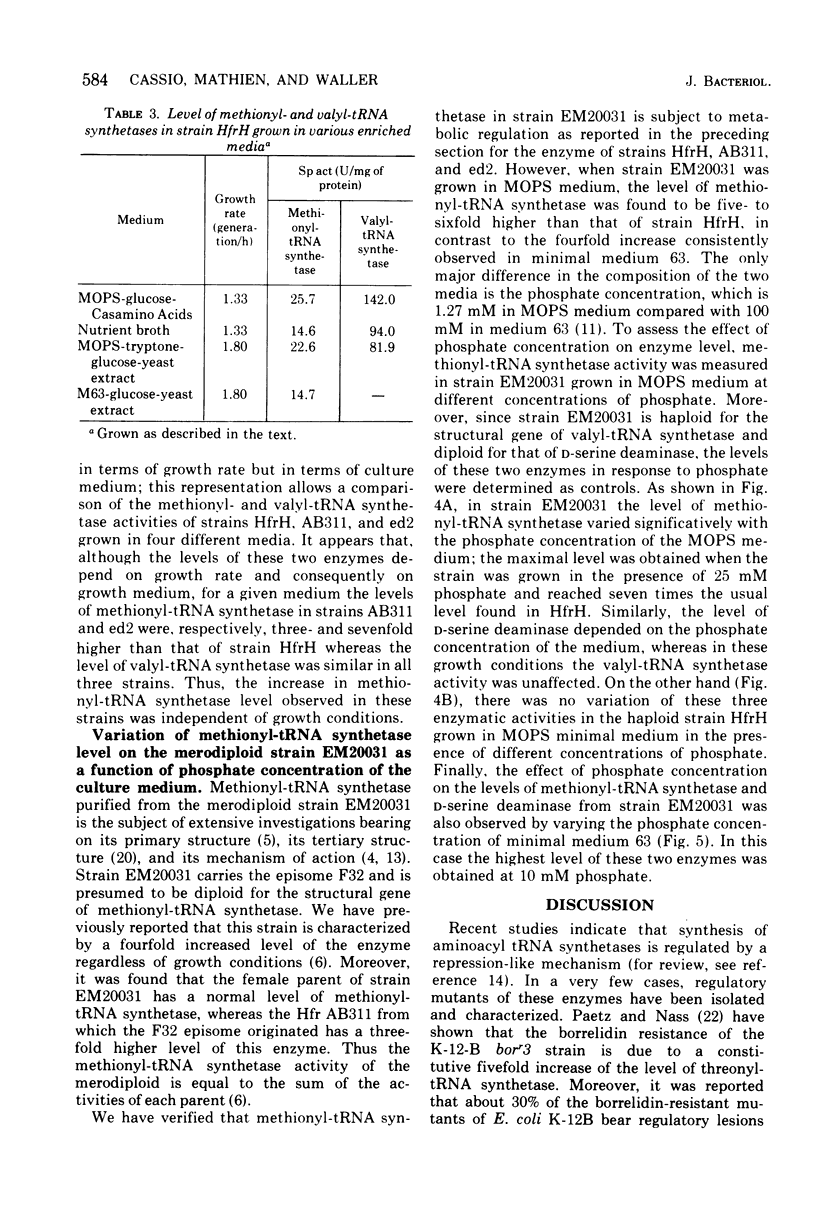
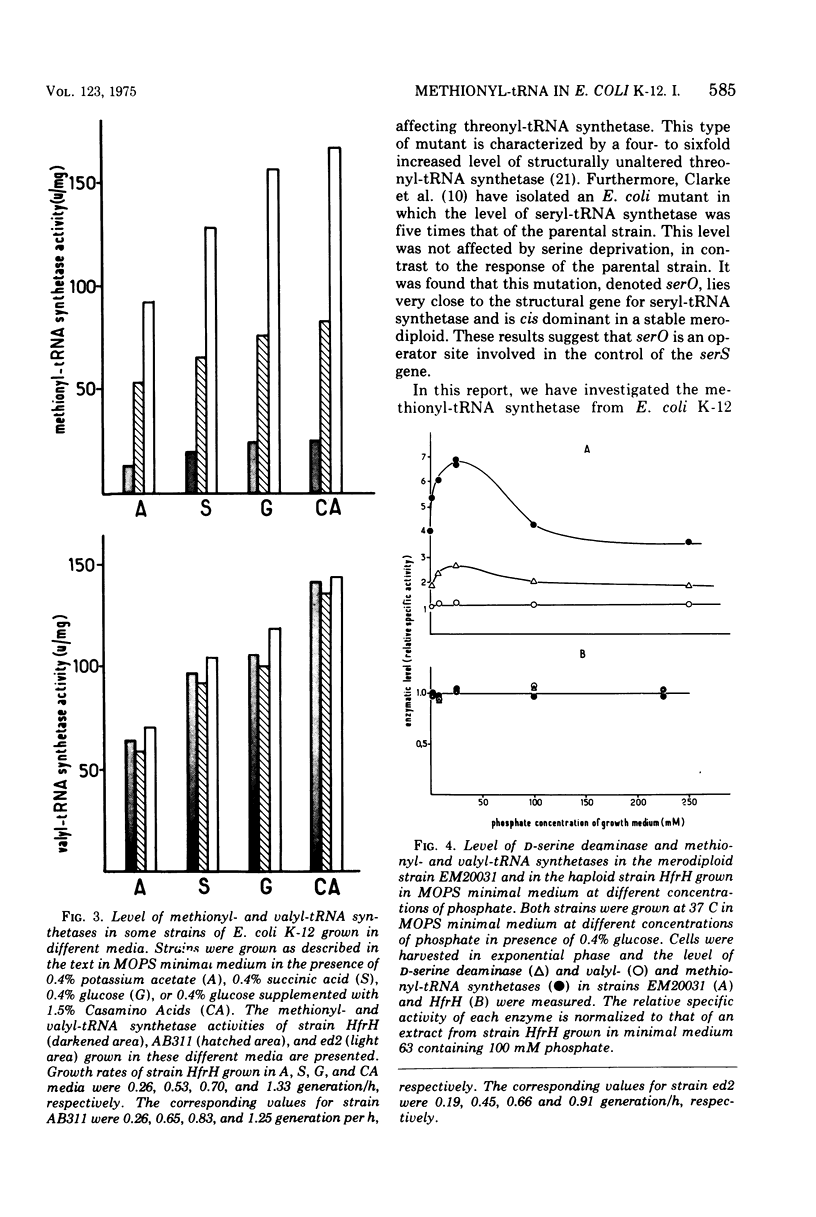
The methionyl-transfer ribonucleic acid (tRNA) synthetase of Escherichia coli K-12 eductants carrying P2-mediated deletions in the region of the structural gene of this enzyme was investigated. No structural alteration of this enzyme was observed in three eductants examined. These were isolated from strain AB311, which had a threefold higher level of methionyl-tRNA synthetase than most haploid strains examined. In two of the three eductants studied, the level of this enzyme was twofold higher than in their parental strain regardless of growth conditions used. In contrast, isoleucyl-, leucyl-, and valyl-tRNA synthetases had similar levels in all strains examined. Like valyl-tRNA synthetase, but to a lesser extent, methionyl-tRNA synthetase was subject to metabolic regulation. Coupling between the level of methionyl-tRNA synthetase and growth rate was observed even in strains that had an enhanced level of methionyl-tRNA synthetase. These results suggest that the formation of methionyl-tRNA synthetase remains subject to metabolic regulation even when the repression-like mechanism that controls the synthesis of this enzyme is altered. In addition, we report that in the merodiploid strain EM20031, which was haploid for the valyl-tRNA synthetase structural gene and diploid for the structural genes of methionyl-tRNA synthetase and D-serine deaminase, the levels of these latter two enzymes varied to a minor yet significant extent with the phosphate concentration of the culture medium; under the same conditions, the level of valyl-tRNA synthetase remained unchanged. Moreover, no variation of the levels of these three enzymes in response to phosphate was observed in the haploid strain HfrH. These results indicate that in the merodiploid strain EM20031, which carries the episome F32, the number of episomes per chromosome varies to some extent according to the phosphate concentration of the culture medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibold E. R., Williams L. S. Regulation of methionyl-transfer ribonucleic acid synthetase formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1973 Jun;114(3):1007–1013. doi: 10.1128/jb.114.3.1007-1013.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquet S., Iwatsubo M., Waller J. P. The mechanism of action of methionyl-tRNA synthetase from Escherichia coli. 1. Fluorescence studies on tRNAMet binding as a function of ligands, ions and pH. Eur J Biochem. 1973 Jul 2;36(1):213–226. doi: 10.1111/j.1432-1033.1973.tb02903.x. [DOI] [PubMed] [Google Scholar]
- Bruton C. J., Jakes R., Koch G. L. Repeated sequences in methionyl-tRNA synthetase from E. coli. FEBS Lett. 1974 Sep 1;45(1):26–28. doi: 10.1016/0014-5793(74)80802-1. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Cassio D., Lawrence F., Lawrence D. A. Level of methionyl-tRNA synthetase in merodiploids of Escherichia coli K12. Eur J Biochem. 1970 Aug;15(2):331–334. doi: 10.1111/j.1432-1033.1970.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Cassio D., Mathien Y. Effect of L-methioninyl adenylate on the level of aminoacylation in vivo of tRNA(Met) from Escherichia coli K12. Nucleic Acids Res. 1974 May;1(5):719–725. doi: 10.1093/nar/1.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassio D., Waller J. -P. Purification and properties of methionyl-tRNA synthetase from E. coli K 12 carrying the F32 episome. FEBS Lett. 1971 Feb 9;12(6):309–312. doi: 10.1016/0014-5793(71)80002-9. [DOI] [PubMed] [Google Scholar]
- Cassio D., Waller J. P. Modification of methionyl-tRNA synthetase by proteolytic cleavage and properties of the trypsin-modified enzyme. Eur J Biochem. 1971 May 28;20(2):283–300. doi: 10.1111/j.1432-1033.1971.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Clarke S. J., Low B., Konigsberg W. Isolation and characterization of a regulatory mutant of an aminoacyl-transfer ribonucleic acid synthetase in Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1096–1103. doi: 10.1128/jb.113.3.1096-1103.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. Relationship of Flac replication and chromosome replication. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2706–2710. doi: 10.1073/pnas.69.9.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayat G., Waller J. P. The mechanism of action of methionyl-tRNA synthetase from Escherichia coli. Equilibrium-dialysis studies on the binding of methionine, ATP and ATP-Mg2+ by the native and trypsin-modified enzymes. Eur J Biochem. 1974 May 15;44(2):335–342. doi: 10.1111/j.1432-1033.1974.tb03490.x. [DOI] [PubMed] [Google Scholar]
- Kisselev L. L., Favorova O. O. Aminoacyl-tRNA synthetases: sone recent results and achievements. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):141–238. doi: 10.1002/9780470122853.ch5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemoine F., Waller J. P., van Rapenbusch R. Studies on methionyl transfer RNA synthetase. 1. Purification and some properties of methionyl transfer RNA synthetase from Escherichia coli K-12. Eur J Biochem. 1968 Apr 3;4(2):213–221. doi: 10.1111/j.1432-1033.1968.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFALL E. GENETIC STRUCTURE OF THE D-SERINE DEAMINASE SYSTEM OF ESCHERICHIA COLI. J Mol Biol. 1964 Sep;9:746–753. doi: 10.1016/s0022-2836(64)80179-0. [DOI] [PubMed] [Google Scholar]
- McFall E. "Position effect" on dominance in the D-serine deaminase system of Escherichia coli K-12. J Bacteriol. 1967 Dec;94(6):1989–1993. doi: 10.1128/jb.94.6.1989-1993.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteilhet C., Zelwer C., Risler J. L. Two isomorphous heavy-atom derivatives of crystalline methionyl-tRNA synthetase from Escherichia coli. FEBS Lett. 1974 Sep 15;46(1):101–105. doi: 10.1016/0014-5793(74)80344-3. [DOI] [PubMed] [Google Scholar]
- Nass G., Thomale J. Alteration of structure of level of threonyl-tRNA-synthetase in Borrelidin resistant mutants of E. coli. FEBS Lett. 1974 Feb 15;39(2):182–186. doi: 10.1016/0014-5793(74)80046-3. [DOI] [PubMed] [Google Scholar]
- Paetz W., Nass G. Biochemical and immunological characterization of threonyl-tRNA synthetase of two borrelidin-resistant mutants of Escherichia coli K12. Eur J Biochem. 1973 Jun;35(2):331–337. doi: 10.1111/j.1432-1033.1973.tb02843.x. [DOI] [PubMed] [Google Scholar]
- Parker J., Flashner M., Mckeever W. G., Neidhardt F. C. Metabolic regulation of the arginyl and valyl transfer ribonucleic acid synthetases in bacteria. J Biol Chem. 1974 Feb 25;249(4):1044–1053. [PubMed] [Google Scholar]
- Parker J., Neidhardt F. C. Metabolic regulation of aminoacyl-tRNA synthetase formation in bacteria. Biochem Biophys Res Commun. 1972 Oct 17;49(2):495–501. doi: 10.1016/0006-291x(72)90438-x. [DOI] [PubMed] [Google Scholar]
- Sunshine M. G., Kelly B. Extent of host deletions associated with bacteriophage P2-mediated eduction. J Bacteriol. 1971 Nov;108(2):695–704. doi: 10.1128/jb.108.2.695-704.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen J., Pato M. L. Replication of the F'lac sex factor in the cell cycle of Escherichia coli. Mol Gen Genet. 1971;111(3):242–255. doi: 10.1007/BF00433109. [DOI] [PubMed] [Google Scholar]



