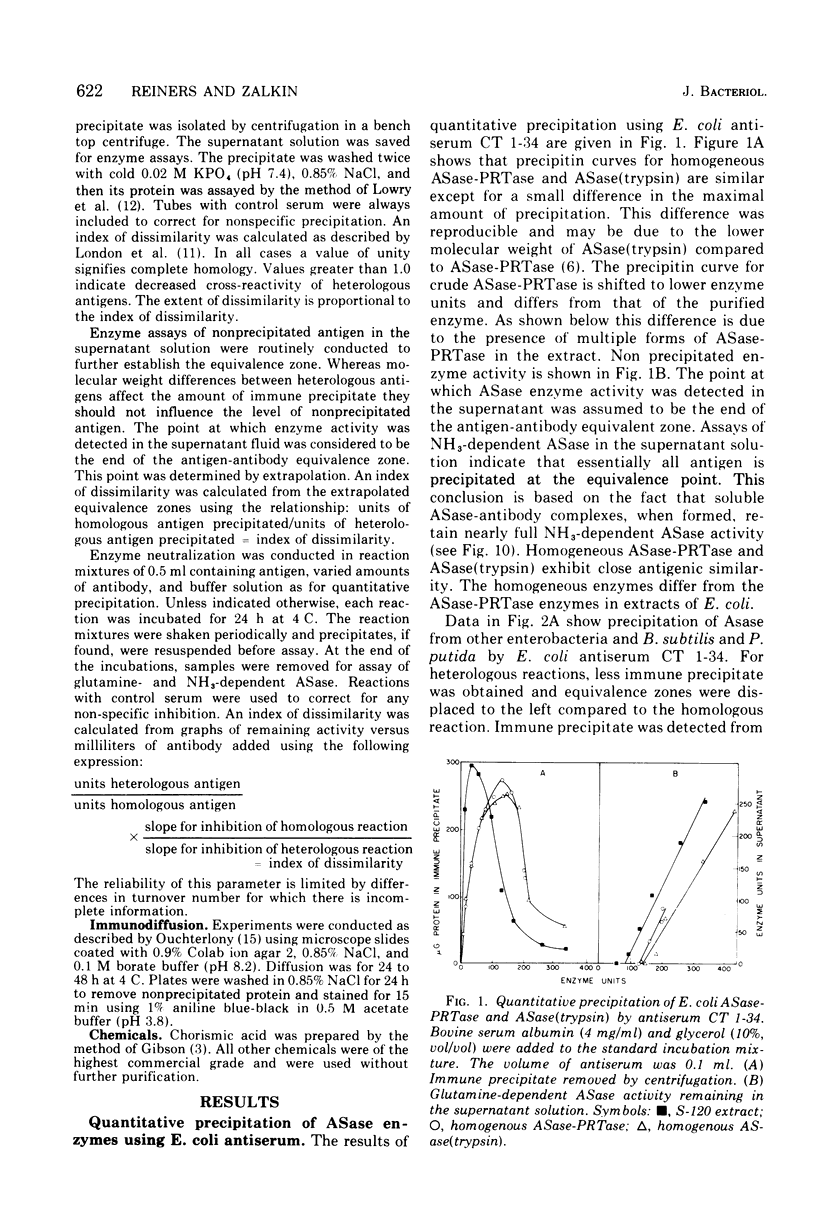
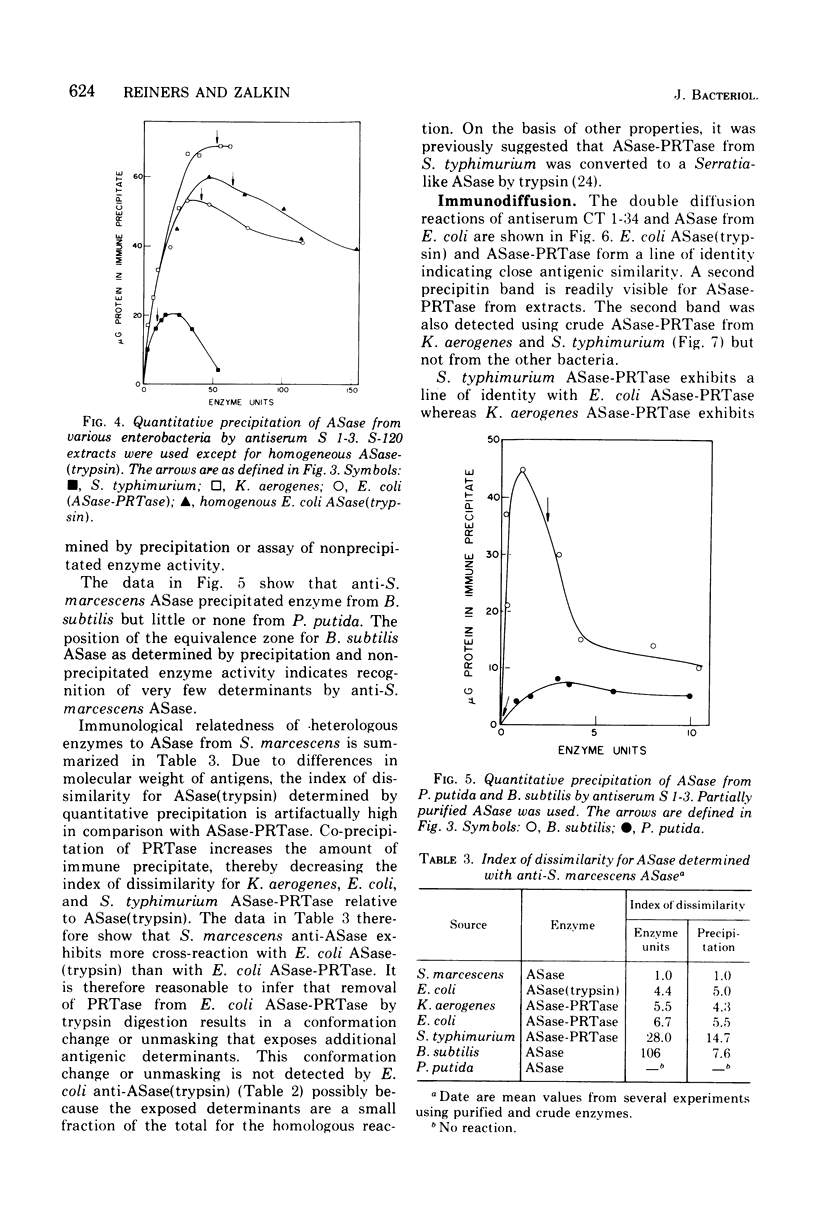
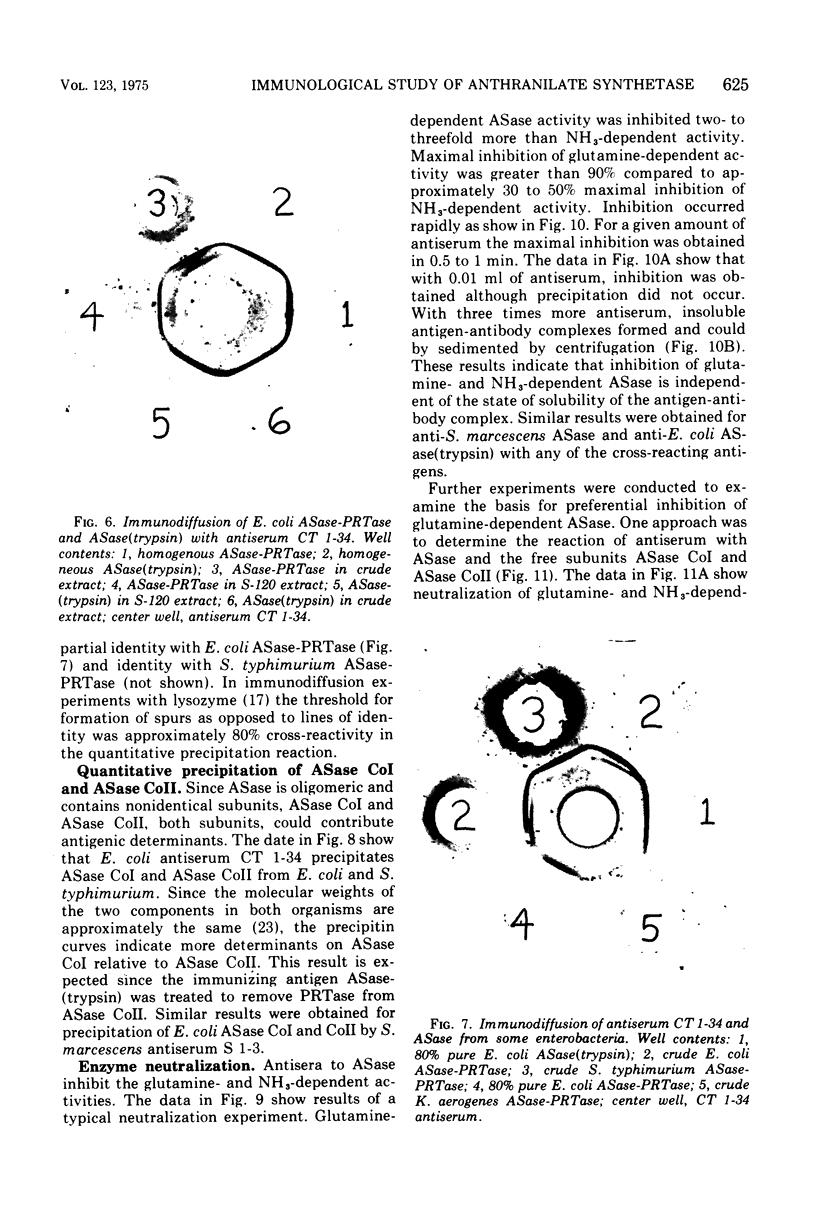
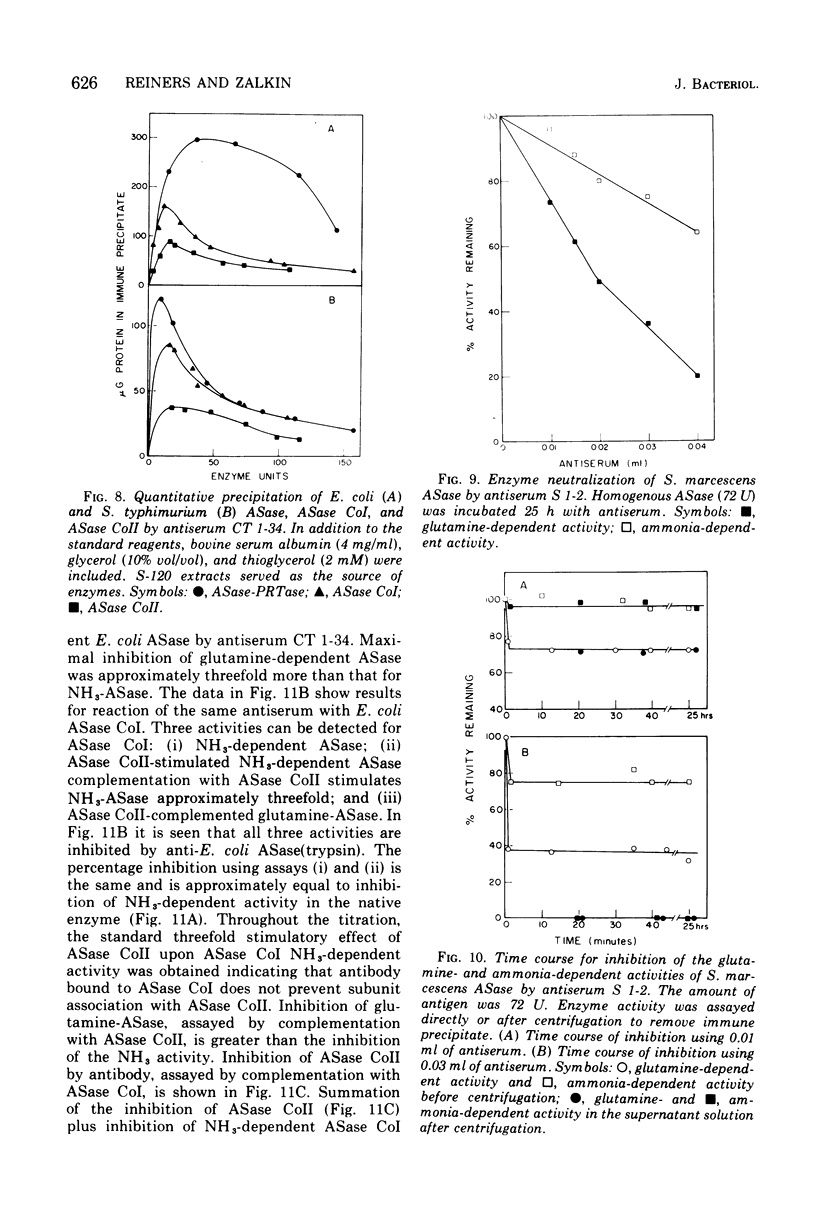
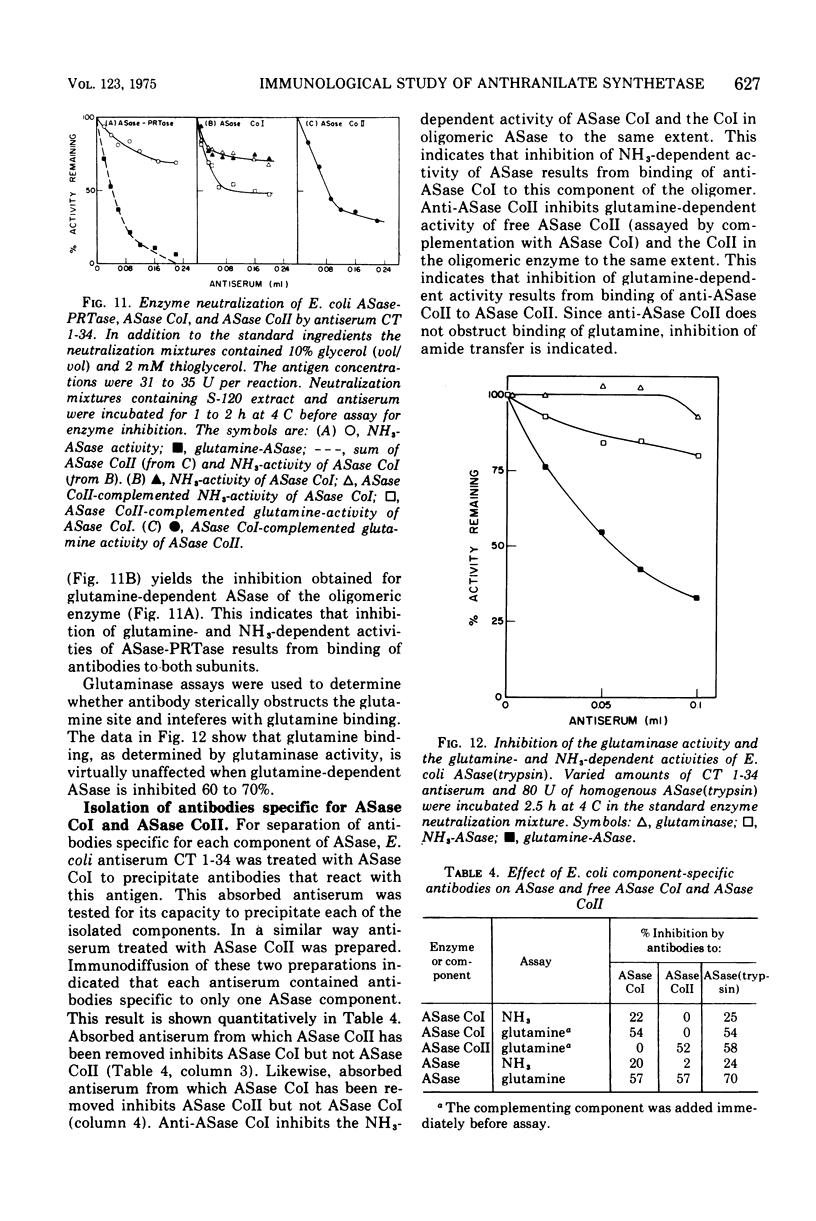
Abstract
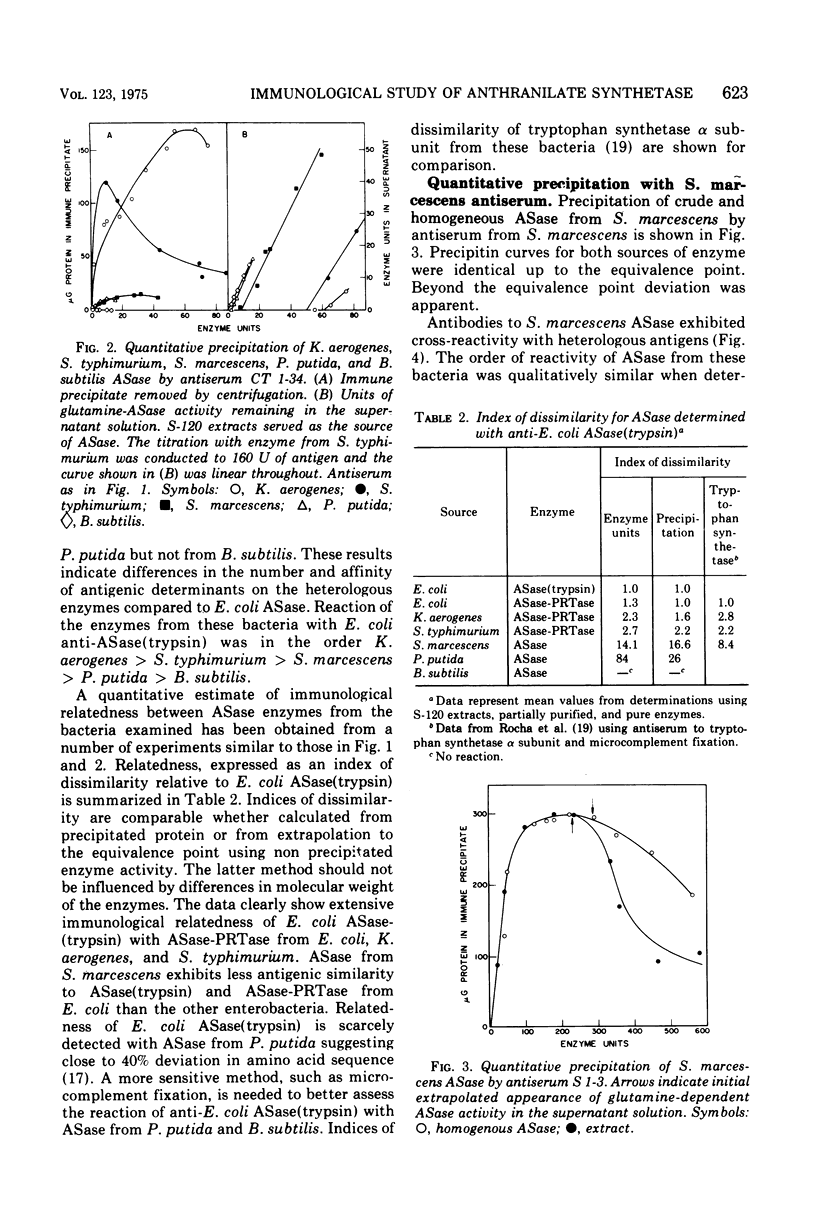
An immunological study of anthranilate synthetase (ASase) has been initiated using quantitative precipitation, enzyme neutralization, and immunodiffusion methods. Cross-reactivity of anthranilate synthetase-anthranilate-5-phosphoribosylpyrophosphate phosphoribosyltransferase (ASase-PRTase) from Escherichia coli, Klebsiella aerogenes, and Salmonella typhimurium and ASase from Serratia marcescens and Pseudomonas putida was detected with antibodies to ?E. coli trypsin-treated ASase. Cross-reactivity of antigens was also obtained with S. marcescens anti-ASase. Indices of dissimilarity verified the overall structural similarity of ASase-PRTase from E. coli, K. aerogenes, and S. typhimurium and the divergence from S. marcescens ASase. Further divergence of these enzymes from ASase in B. subtilis and P. putida was apparent. Precipitation of ASase components I and II (ASase CoI and ASase CoII) was obtained using anti-ASase or antiserum fractionated to contain component-specific antibodies. Anti-ASase inhibited enzyme activity to binding to determinants on both subunits. Anti-ASase CoI inhibited the ammonia-dependent reaction and interfered with amide transfer from glutaminyl-ASase CoII. Anti-ASase CoII inhibited the glutamine reaction by blocking amide transfer. Enzyme neutralization experiments indicate more conservation of determinants at the active site region of ASase CoII compared to ASase CoI in the enterobacteria. A particulate form of ASase-PRTase in E. coli, K. aerogenes, and S. typhimurium could be distinguished by quantitative precipitation and immunodiffusion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Henderson E. J., Nagano H., Zalkin H., Hwang L. H. The anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase aggregate. Purification of the aggregate and regulatory properties of anthranilate synthetase. J Biol Chem. 1970 Mar 25;245(6):1416–1423. [PubMed] [Google Scholar]
- Henderson E. J., Zalkin H., Hwang L. H. The anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase aggregate. Catalytic and regulatory properties of aggregated and unaggregated forms of anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase. J Biol Chem. 1970 Mar 25;245(6):1424–1431. [PubMed] [Google Scholar]
- Hwang L. H., Zalkin H. Multiple forms of anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase from Salmonella typhimurium. J Biol Chem. 1971 Apr 25;246(8):2338–2345. [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Localization of two functions of the phosphoribosyl anthranilate transferase of Escherichia coli to distinct regions of the polypeptide chain. J Bacteriol. 1974 Feb;117(2):502–508. doi: 10.1128/jb.117.2.502-508.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. F., Homes W. M., Smiley K. L., Jr, Jensen R. A. Rapid regulation of an anthranilate synthase aggregate by hysteresis. J Bacteriol. 1973 Jan;113(1):224–232. doi: 10.1128/jb.113.1.224-232.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li S. L., Hanlon J., Yanofsky C. Separation of anthranilate synthetase components I and II of Escherichia coli, Salmonella typhimurium, and Serratia marcescens and determination of their amino-terminal sequences by automatic Edman degradation. Biochemistry. 1974 Apr 9;13(8):1736–1744. doi: 10.1021/bi00705a028. [DOI] [PubMed] [Google Scholar]
- Li S. L., Hanlon J., Yanofsky C. Structural homology of the glutamine amidotransferase subunits of the anthranilate synthetases of Escherichia coli, Salmonella typhimurium and Serratia marcescens. Nature. 1974 Mar 1;248(5443):48–50. doi: 10.1038/248048a0. [DOI] [PubMed] [Google Scholar]
- London J., Meyer E. Y., Kulczyk S. Comparative biochemical and immunological study of malic enzyme from two species of lactic acid bacteria: evolutionary implications. J Bacteriol. 1971 Apr;106(1):126–137. doi: 10.1128/jb.106.1.126-137.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano H., Zalkin H., Henderson E. J. The anthranilate synthetase-anthranilate-5-phosphorribosylpyrophosphate phosphoribosyltransferase aggregate. On the reaction mechanism of anthranilate synthetase from Salmonella typhimurium. J Biol Chem. 1970 Aug 10;245(15):3810–3820. [PubMed] [Google Scholar]
- Nagano H., Zalkin H. Some physicochemical properties of anthranilate synthetase component I from Salmonella typhimurium. J Biol Chem. 1970 Jun;245(12):3097–3103. [PubMed] [Google Scholar]
- Patel N., Holmes W. M., Kane J. F. Homologous and hybrid complexes of anthranilate synthase from Bacillus species. J Bacteriol. 1974 Jul;119(1):220–227. doi: 10.1128/jb.119.1.220-227.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. II. Comparison of precipitin and micro-complement fixation results. J Biol Chem. 1971 Nov 25;246(22):7010–7017. [PubMed] [Google Scholar]
- Queener S. W., Queener S. F., Meeks J. R., Gunsalus I. C. Anthranilate synthase from Pseudomonas putida. Purification and properties of a two-component enzyme. J Biol Chem. 1973 Jan 10;248(1):151–161. [PubMed] [Google Scholar]
- Rocha V., Crawford I. P., Mills S. E. Comparative immunological and enzymatic study of the tryptophan synthetase beta 2 subunit in the Enterobacteriaceae. J Bacteriol. 1972 Jul;111(1):163–168. doi: 10.1128/jb.111.1.163-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WEIGLE W. O. Immunochemical properties of the cross-reactions between anti-BSA and heterologous albumins. J Immunol. 1961 Nov;87:599–607. [PubMed] [Google Scholar]
- Zalkin H. Anthranilate synthetase. Adv Enzymol Relat Areas Mol Biol. 1973;38:1–39. doi: 10.1002/9780470122839.ch1. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Kling D. Anthranilate synthetase. Purification and properties of component I from Salmonella typhimurium. Biochemistry. 1968 Oct;7(10):3566–3573. doi: 10.1021/bi00850a034. [DOI] [PubMed] [Google Scholar]