Abstract
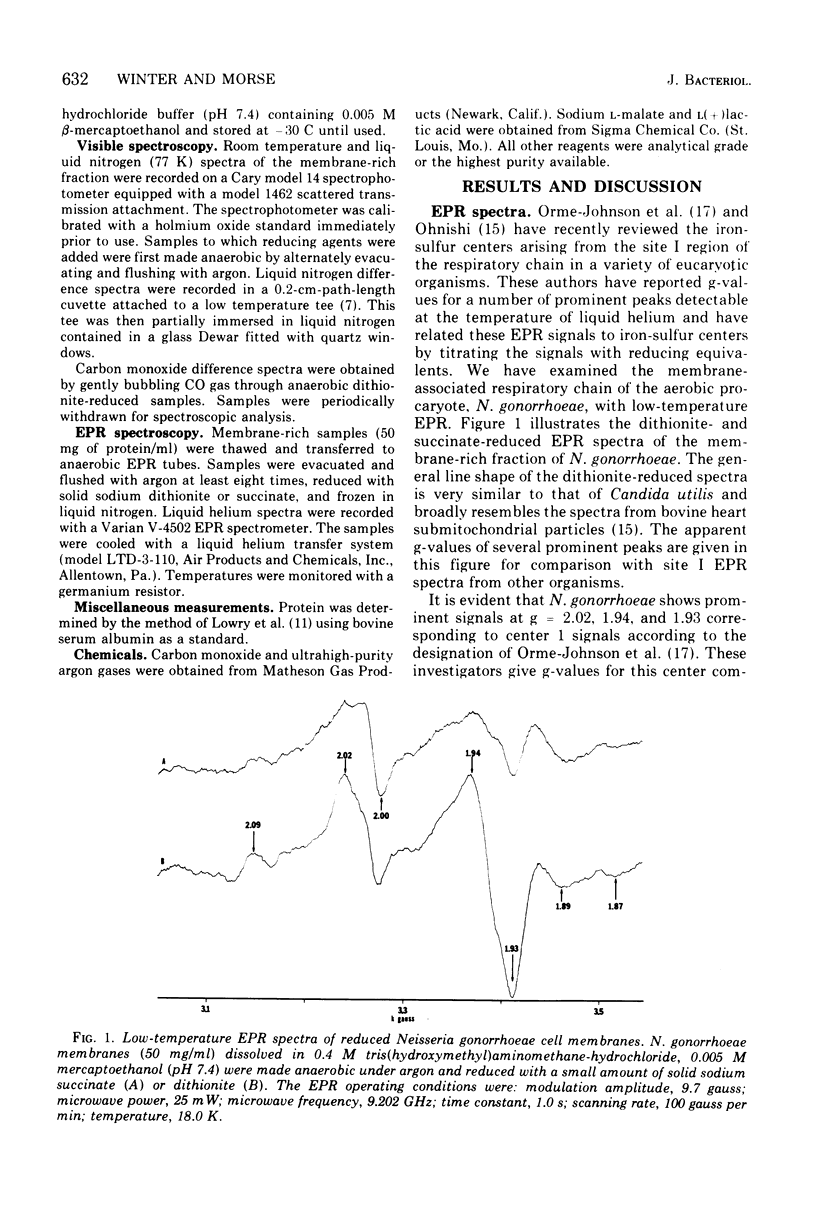
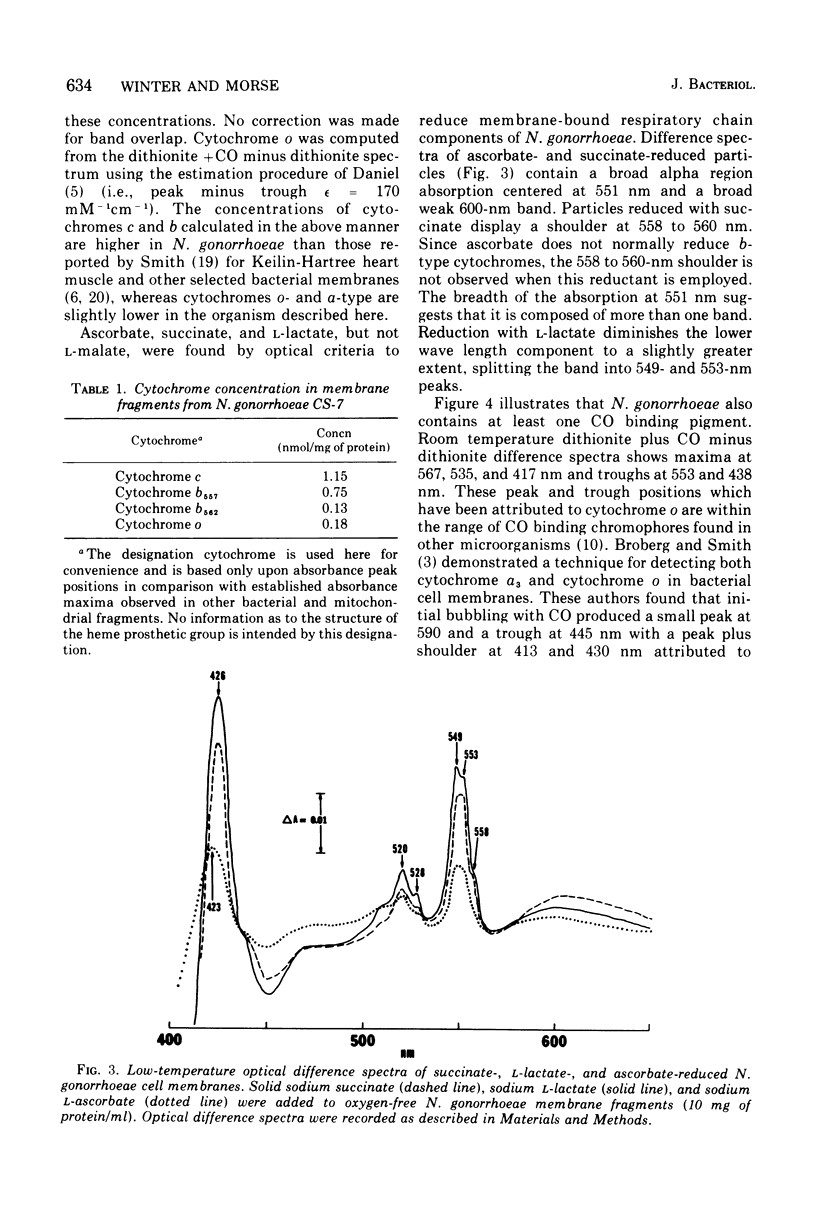
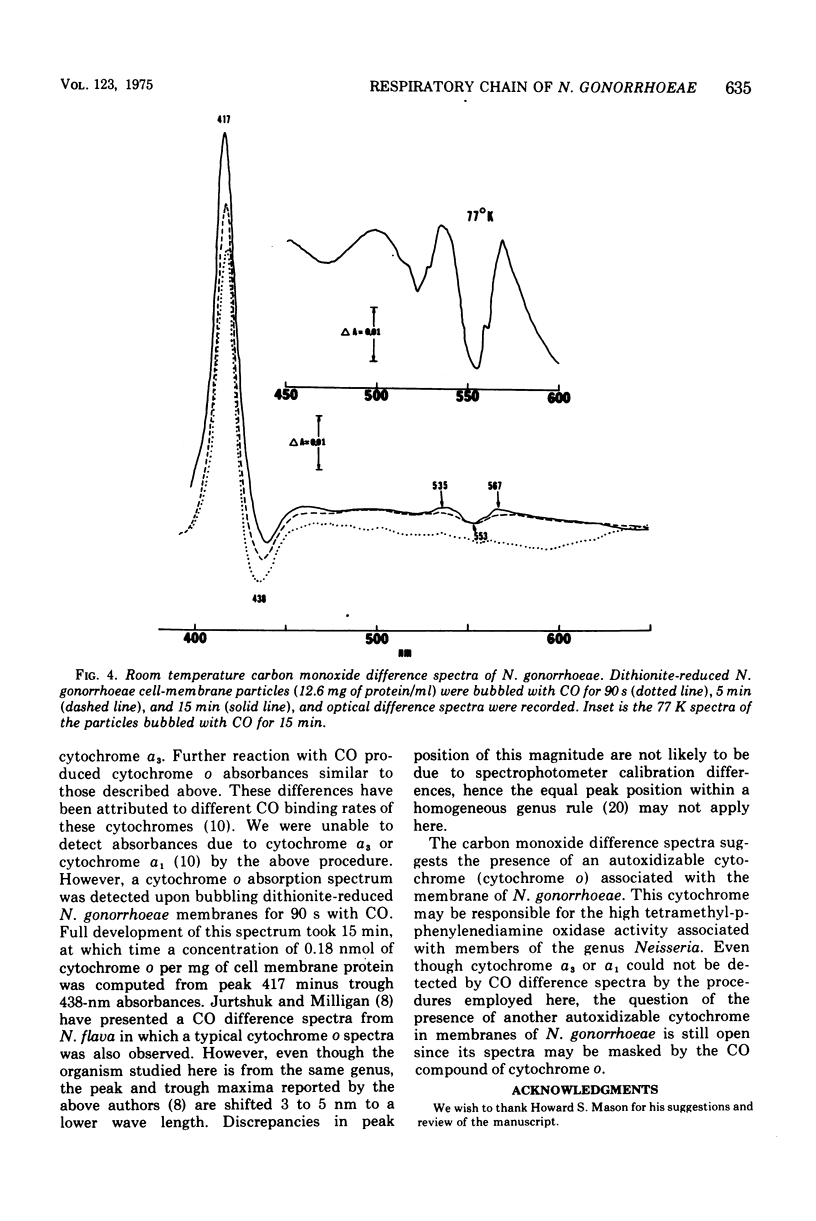
The cell membrane-associated respiratory electron transport chain of Neisseria gonorrhoeae was examined using electron paramagnetic spectroscopy (EPR) at liquid helium temperatures and optical spectroscopy at liquid nitrogen and room temperatures. EPR spectra of dithionite-reduced particles indicated the presence of centers N-1 and N-3 in the site I region of the respiratory chain, whereas reduction with succinate revealed the existence of center S-1 from the succinate cytochrome c reductase segment. Free radical(s) resembling that due to falvin semiquinone were observed with both reductants. Low temperature (77 K) optical difference spectra indicated the presence of cytochromes with alpha band maxima at 549, 557, and 562. Bands at 567, 535, and 417 nm, characteristic of the CO compound of cytochrome o, were also identified. Cytochromes a1 and a3 were not detected; however, a broad but weak absorbance with an alpha band maximun at 600 nm and a Soret shoulder at 440 nm was observed. Hence the respiratory chain of N. gonorrhoeae appears to contain several nonheme iron centers, cytochrome c, two b cytochromes, with cytochrome o which probably serves as the terminal oxidase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H. EPR studies on the mechanism of action of succinate dehydrogenase in activated preparations. Biochem Biophys Res Commun. 1974 Jun 4;58(3):564–572. doi: 10.1016/s0006-291x(74)80457-2. [DOI] [PubMed] [Google Scholar]
- CATLIN B. W., CUNNINGHAM L. S. Transforming activities and base contents of deoxyribonucleate preparations from various Neisseriae. J Gen Microbiol. 1961 Oct;26:303–312. doi: 10.1099/00221287-26-2-303. [DOI] [PubMed] [Google Scholar]
- Daniel R. M. The electron transport system of Acetobacter suboxydans with particular reference to cytochrome. Biochim Biophys Acta. 1970 Sep 1;216(2):328–341. doi: 10.1016/0005-2728(70)90224-0. [DOI] [PubMed] [Google Scholar]
- Dietrich W. E., Jr, Biggins J. Respiratory mechanisms in the Flexibacteriaceae: terminal oxidase systems of Saprospira grandis and Vitreoscilla species. J Bacteriol. 1971 Mar;105(3):1083–1089. doi: 10.1128/jb.105.3.1083-1089.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Milligan T. W. Preliminary characterization studies on the Neisseria catarrhalis respiratory electron transport chain. J Bacteriol. 1974 Oct;120(1):552–555. doi: 10.1128/jb.120.1.552-555.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Milligan T. W. Quantitation of the tetramethyl-p-phenylenediamine oxidase reaction in Neisseria species. Appl Microbiol. 1974 Dec;28(6):1079–1081. doi: 10.1128/am.28.6.1079-1081.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Fitzgerald T. J. Effect of progesterone on Neisseria gonorrhoeae. Infect Immun. 1974 Dec;10(6):1370–1377. doi: 10.1128/iai.10.6.1370-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi T. Mechanism of electron transport and energy conservation in the site I region of the respiratory chain. Biochim Biophys Acta. 1973 Dec 7;301(2):105–128. [PubMed] [Google Scholar]
- Onishi T., Winter D. B., Lim J., King T. E. Low temperature electron paramagnetic resonance studies on two iron-sulfur centers in cardiac succinate dehydrogenase. Biochem Biophys Res Commun. 1973 Jul 2;53(1):231–237. doi: 10.1016/0006-291x(73)91424-1. [DOI] [PubMed] [Google Scholar]
- Spicher G. Cytochromabsorptionsspektren von Bakterien als Hilfsmittel zur Klärung taxonomischer Fragen. 1. Entiwicklung und Beschreibung einer Methode zur Ermittlung der Redox- und Kohlenoxiddifferenzspektren von Suspensionen lebender Bakterienzellen. Zentralbl Bakteriol Orig A. 1974 Jun;226(4):524–540. [PubMed] [Google Scholar]



