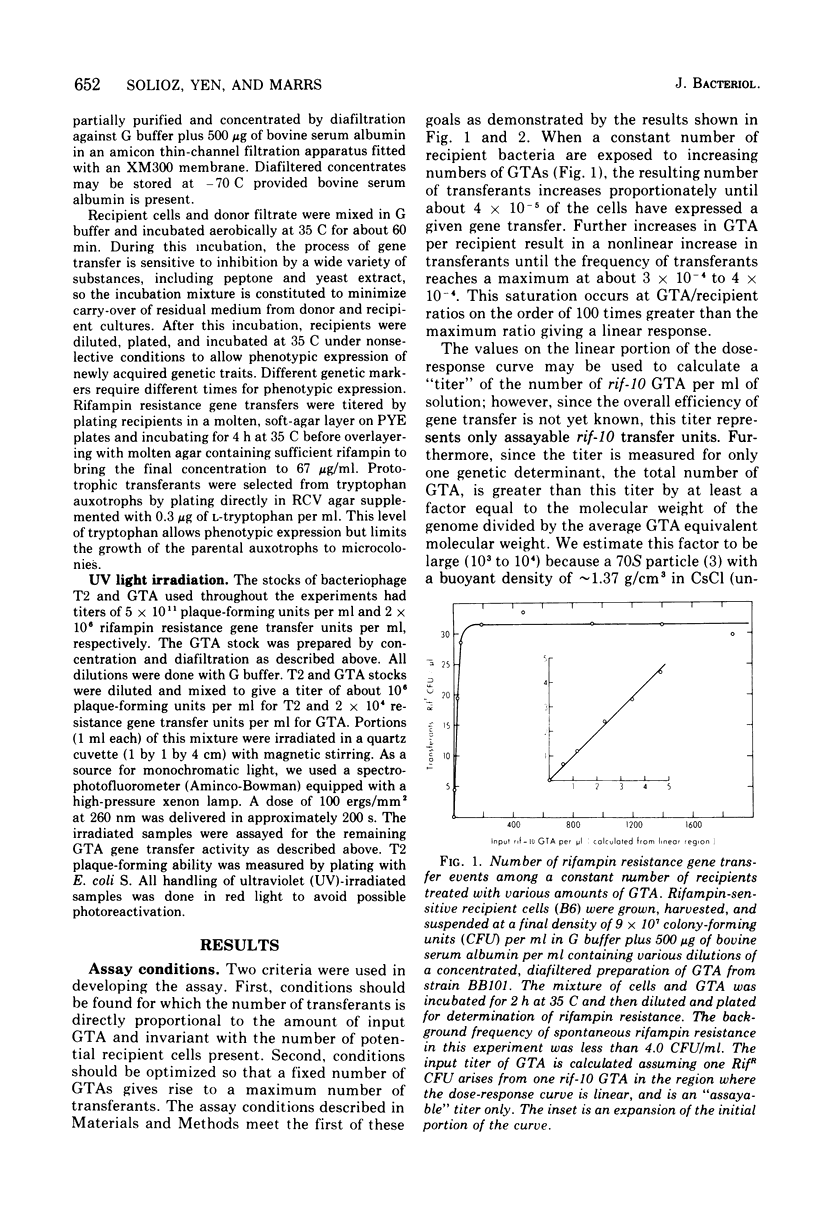
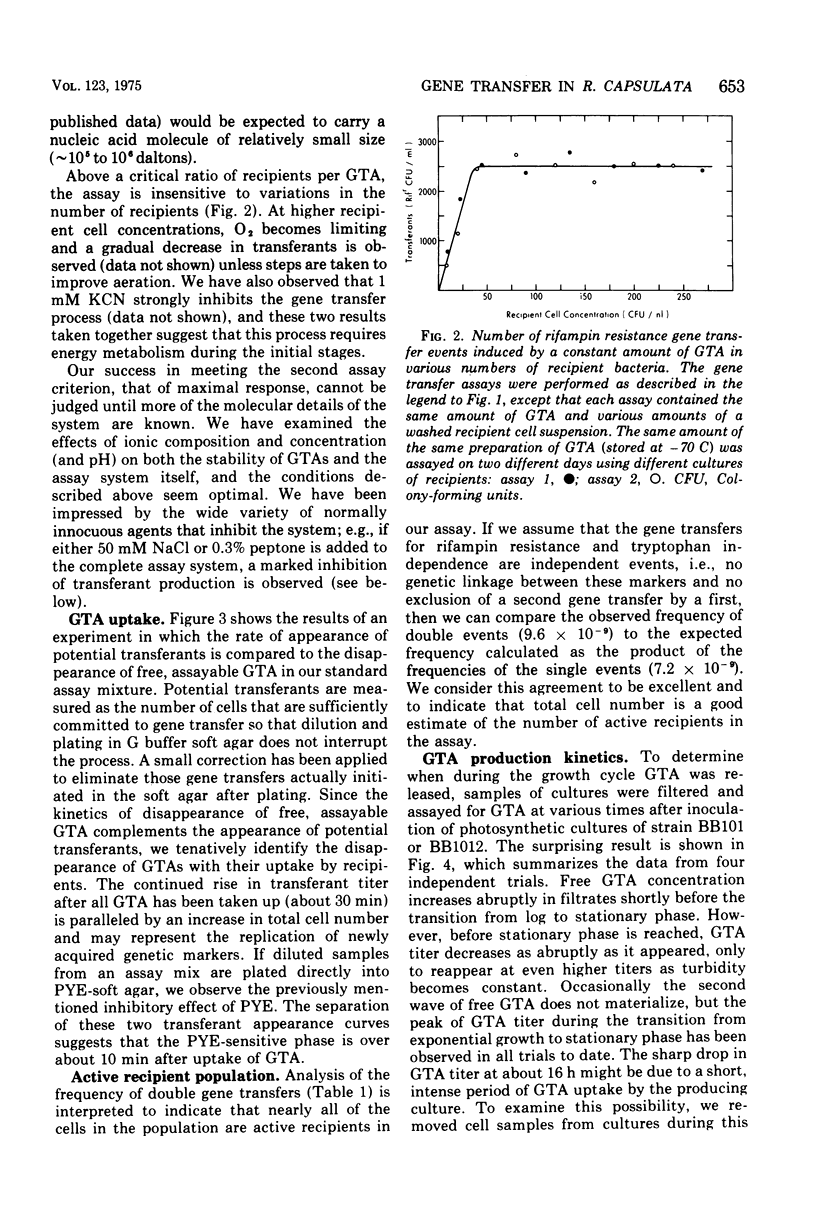
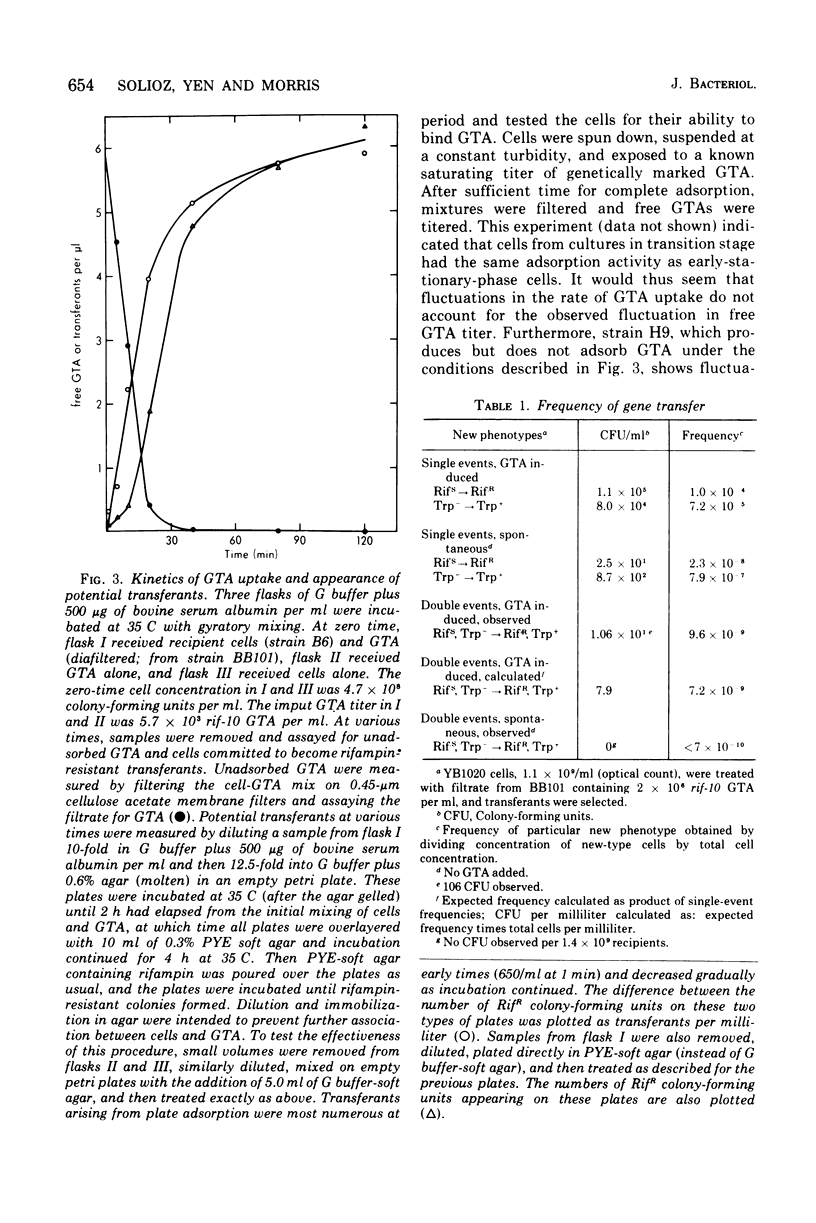
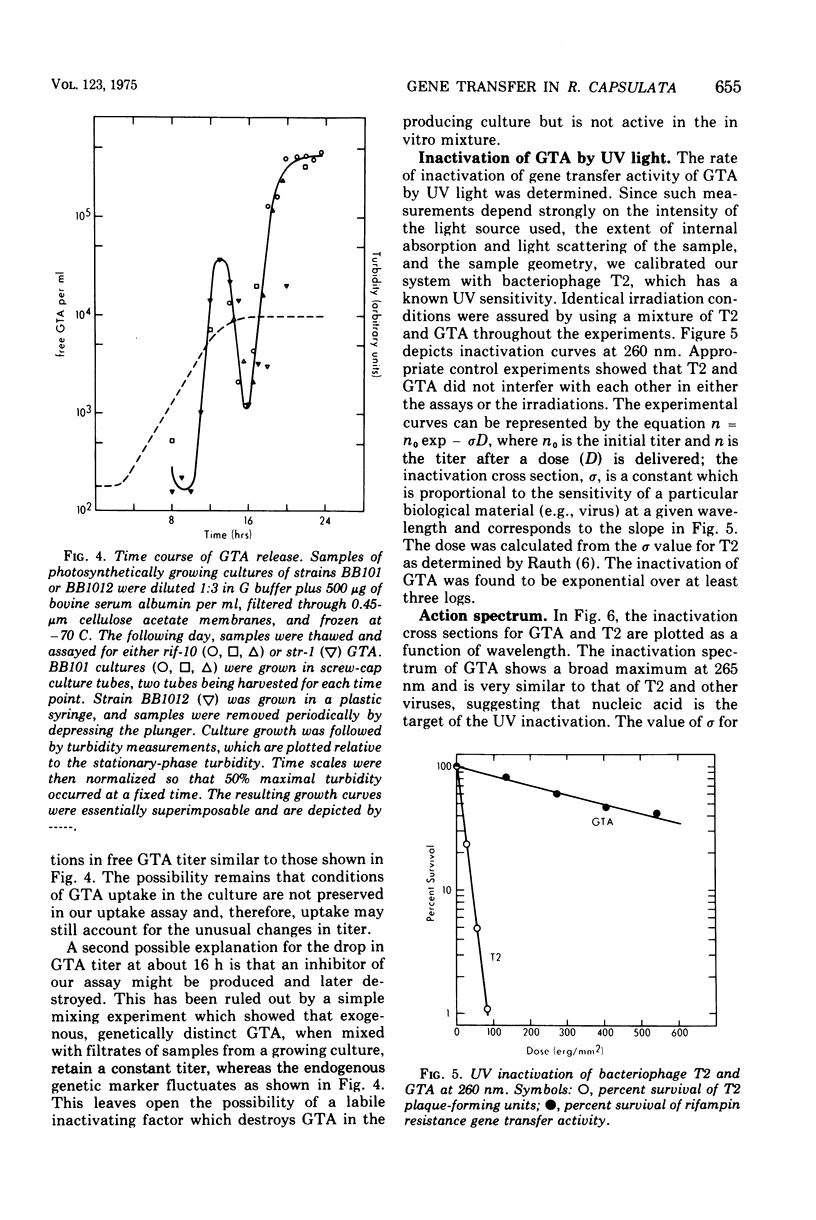
Abstract
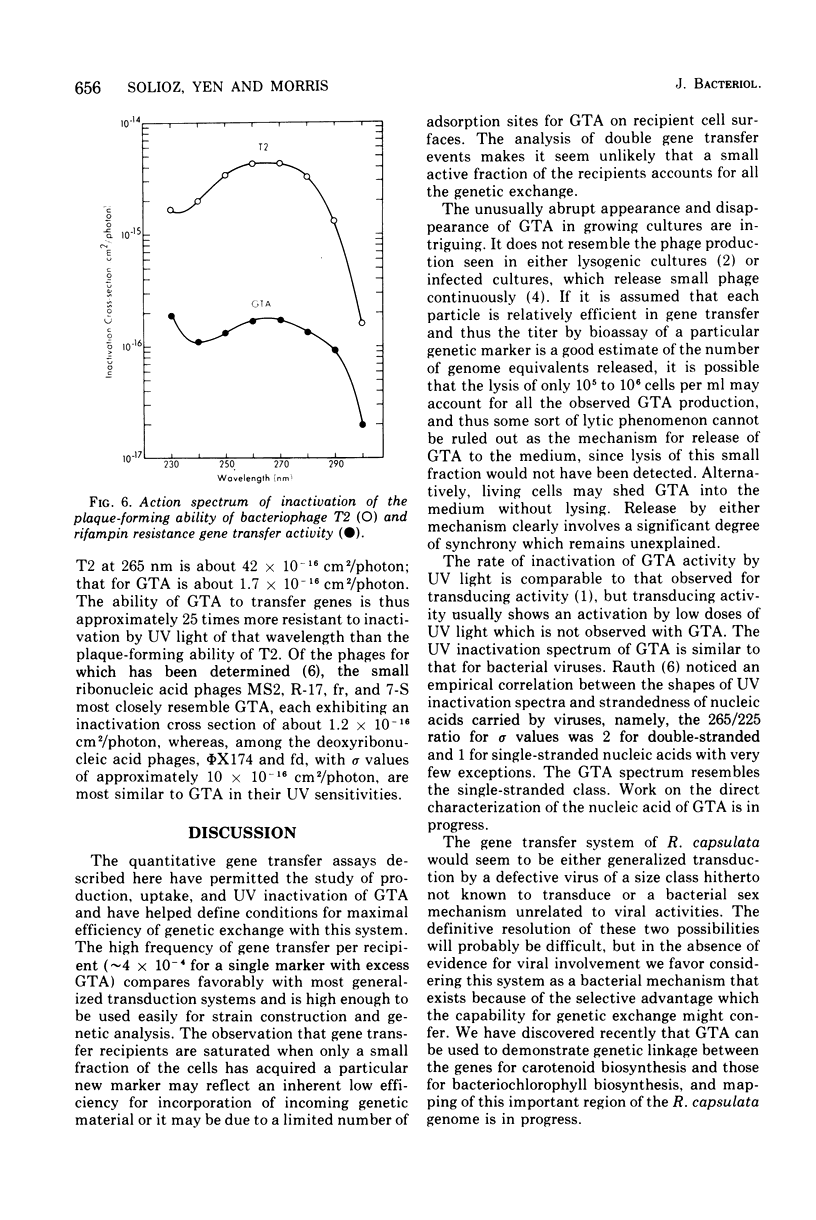
Many strains of Rhodopseudomonas capsulata are capable of exchanging genetic information via a recently discovered gene transfer process involving the release and subsequent uptake from the medium of particles containing genetic information (gene transfer agents, GTAs). No viral activities are observed to be associated with this system. An assay has been developed to quantitate gene transfer in R. capsulata. Conditions are described for which the number of cells acquiring a new genetic trait is direcly proportional to the number of GTAs and independent of the number of receipient cells. These conditions were used for the assay of the uptake and release of GTAs by cells. The maximum fraction of recipients that acquire a given genetic marker is approximately 4 X 10(-4). Free GTA appears in a growing culture in one or two abrupt waves near the time of transition from exponential to stationary phase. During these waves, the titer of GTA for a given marker may reach 2 X 103/ml. A comparison of the frequency of single- and double-marker transfers suggests that most of the cells in early-stationary-phase cultures are active recipients. The ultraviolet inactivation spectrum of GTA resembles that of the small ribonucleic acid phages. The inactivation cross section section beta, for GTA was calculated to be 1.7 X 10(-16) cm2/photon at 265 nm.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENZINGER R., HARTMAN P. E. Effects of ultraviolet light on transducing phage P22. Virology. 1962 Dec;18:614–626. doi: 10.1016/0042-6822(62)90064-8. [DOI] [PubMed] [Google Scholar]
- Marrs B. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1974 Mar;71(3):971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Rauth A. M. The Physical State of Viral Nucleic Acid and the Sensitivity of Viruses to Ultraviolet Light. Biophys J. 1965 May;5(3):257–273. doi: 10.1016/s0006-3495(65)86715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]



