Abstract
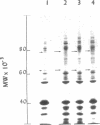
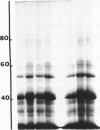
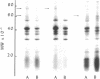
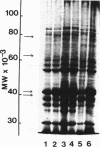
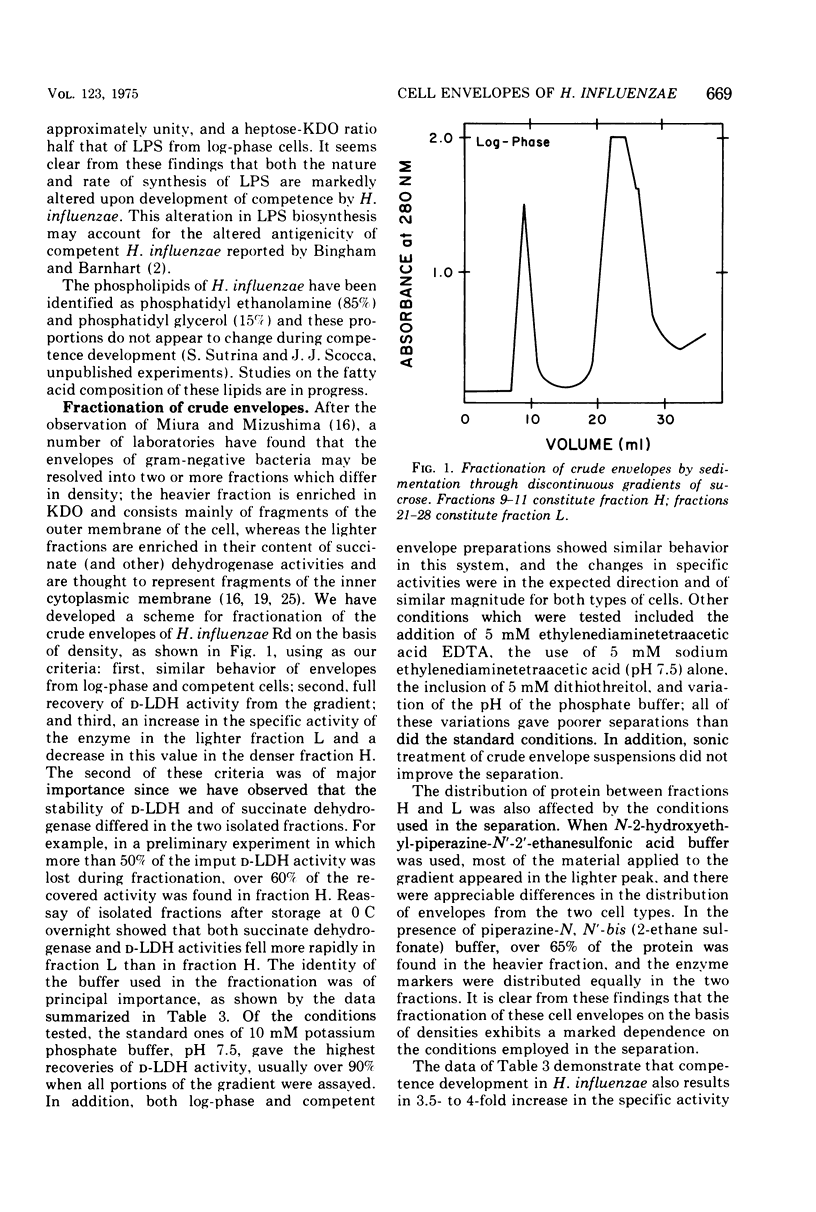
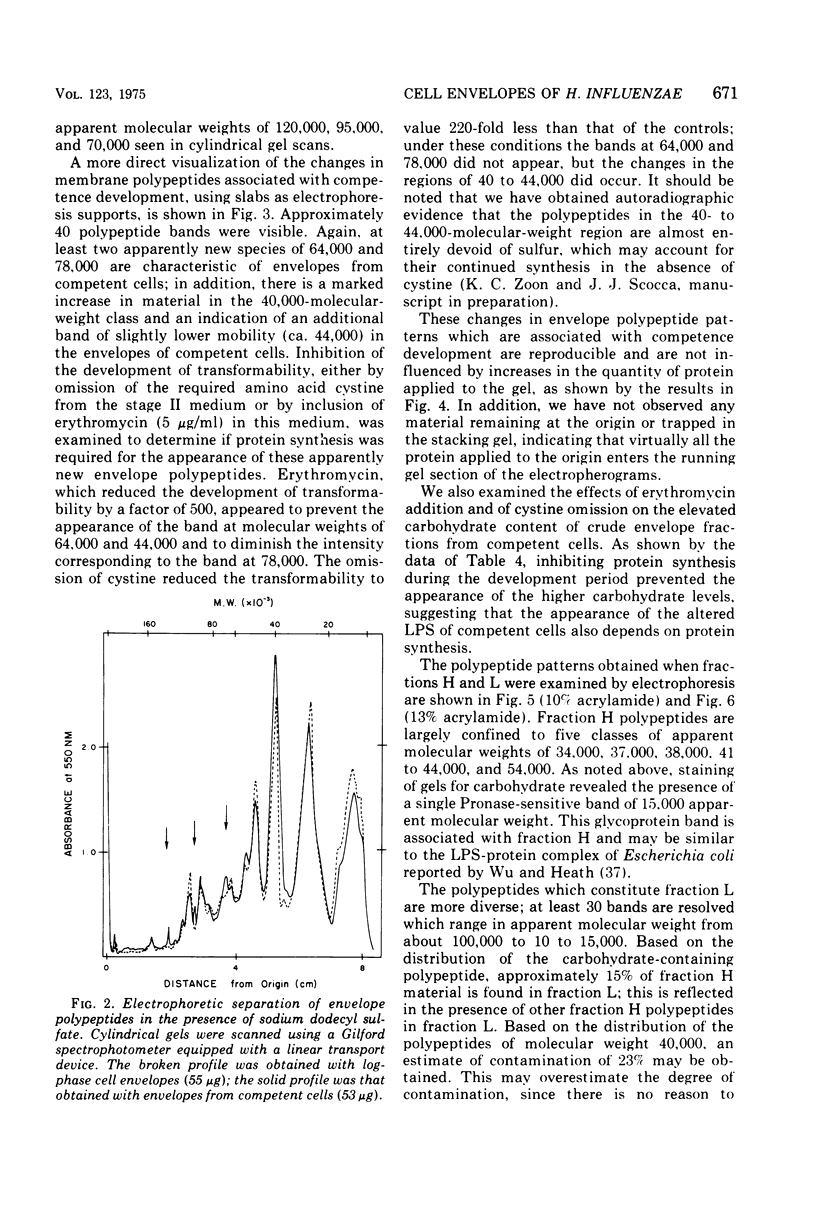
Cell envelopes of Haemophilus influenzae have been prepared by breakage in a French pressure cell followed by differential centrifugation. The envelope fraction may be resolved into an inner-membrane (light) and an outer-membrane (heavy) fraction on density gradients. Envelopes from competent cells possess elevated levels of lipopolysaccharide with a composition different from that of log-phase cell envelopes. Three apparently new polypeptides have been observed in envelopes from competent cells by gel electrophoresis in sodium dodecyl sulfate; additional quantitative alterations in the profiles of membrane polypeptides also company the development of the capacity to transport deoxyribonucleic acid. Most of the polypeptide changes are confined to the outer membrane; one new polypeptide is associated with the inner cytoplasmic membrane of competent cells. Protein synthesis during competence developement is rquired for the change in lipopolysaccharides and in the envelope polypeptides to occur.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch L. L., Bonsen P. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIV. Phospholipids in Bacillus megaterium. J Bacteriol. 1969 Apr;98(1):75–81. doi: 10.1128/jb.98.1.75-81.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham D. P., Barnhart B. J. Inhibition of transformation by antibodies against competent Haemophilus influenzae. J Gen Microbiol. 1973 Apr;75(2):249–258. doi: 10.1099/00221287-75-2-249. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Lange R., Willecke K. Competent Bacillus subtilis cultures synthesize a denatured DNA binding activity. Proc Natl Acad Sci U S A. 1975 Jan;72(1):323–327. doi: 10.1073/pnas.72.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Yee M. L. Homogeneity of envelope proteins of Escherichia coli separated by gel electrophoresis in sodium dodecyl sulfate. J Bacteriol. 1973 Jan;113(1):304–312. doi: 10.1128/jb.113.1.304-312.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKelvy J. F., Lee Y. C. Microheterogeneity of the carbohydrate group of aspergillus oryzae alpha-amylase. Arch Biochem Biophys. 1969 Jun;132(1):99–110. doi: 10.1016/0003-9861(69)90341-5. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Huang P. C. Identification of competence-repressing factors during log-phase growth of Haemophilus influenzae. J Bacteriol. 1972 Feb;109(2):560–564. doi: 10.1128/jb.109.2.560-564.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K. Mechanism of bacterial transformation and transfection. Prog Nucleic Acid Res Mol Biol. 1974;14(0):39–100. doi: 10.1016/s0079-6603(08)60205-6. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Ranhand J. M. Inhibition of the development of competence in Streptococcus sanguis (Wicky) by reagents that interact with sulfhydryl groups: discernment of the competence process. J Bacteriol. 1974 Jun;118(3):1041–1050. doi: 10.1128/jb.118.3.1041-1050.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhand J. M., Lichstein H. C. Effect of selected antibiotics and other inhibitors on competence development in Haemophilus influenzae. J Gen Microbiol. 1969 Jan;55(1):37–43. doi: 10.1099/00221287-55-1-37. [DOI] [PubMed] [Google Scholar]
- Ranhand J. M., Theodore T. S., Cole R. M. Protein difference between competent and noncompetent cultures of a group H Streptococcus. J Bacteriol. 1970 Oct;104(1):360–362. doi: 10.1128/jb.104.1.360-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodden J. L., Scocca J. J. Purification and properties of cyclic phosphodiesterase: 3'-nucleotidase, a periplasmic enzyme of Haemophilus influenzae. Arch Biochem Biophys. 1972 Dec;153(2):837–844. doi: 10.1016/0003-9861(72)90406-7. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. II. Heterogeneity of major outer membrane polypeptides. Arch Biochem Biophys. 1973 Aug;157(2):553–560. doi: 10.1016/0003-9861(73)90674-7. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scocca J. J., Poland R. L., Zoon K. C. Specificity in deoxyribonucleic acid uptake by transformable Haemophilus influenzae. J Bacteriol. 1974 May;118(2):369–373. doi: 10.1128/jb.118.2.369-373.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Tomasz A. Protoplast formation and leakage of intramembrane cell components: induction by the competence activator substance of pneumococci. J Bacteriol. 1975 Jan;121(1):344–353. doi: 10.1128/jb.121.1.344-353.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wise E. M., Jr, Alexander S. P., Powers M. Adenosine 3':5'-cyclic monophosphate as a regulator of bacterial transformation. Proc Natl Acad Sci U S A. 1973 Feb;70(2):471–474. doi: 10.1073/pnas.70.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. C., Heath E. C. Isolation and characterization of lipopolysaccharide protein from Escherichia coli. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2572–2576. doi: 10.1073/pnas.70.9.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]