Abstract
Nicotinamide adenine dinucleotide-linked malate dehydrogenase has been purified from Pseudomonas testosteroni (ATCC 11996). The purification represents over 450-fold increase in specific activity. The amino acid composition of the enzyme was determined and found to be quite different from the composition of the malate dehydrogenases from animal sources as well as from Escherichia coli. Despite this difference, however, the data show that the enzymatic properties of the purified enzyme are remarkably similar to those of other malate dehydrogenases that have been previously studied. The Pseudomonas enzyme has a molecular weight of 74,000 and consists of two subunits of identical size. In addition to L-malate, the enzyme slowly oxidizes other four-carbon dicarboylates having an alpha-hydroxyl group of S configuration such as meso- and (-) tartrate. Rate-determining steps, which differ from that of the reaction involving L-malate, are discussed for the reaction involving these alternative substrates. Oxidation of hydroxymalonate, a process previously undetected with other malate dehydrogenases, is demonstrated fluorometrically. Hydroxymalonate and D-malate strongly enhance the fluorescence of the reduced nicotinamide adenine dinucleotide bound to the enzyme. The enzyme is A-stereospecific with respect to the coenzyme. Malate dehydrogenase is present in a single form in the Pseudomonas. The susceptibility of the enzyme to activation or inhibition by its substrates-particularly the favoring of the oxidation of malate at elevated concentrations-strongly resembles the properties of the mitochondrial enzymes. The present study reveals that whereas profound variations in chemical composition have occurred between the prokaryotic and eukaryotic enzymes, the physical and catalytic properties of malate dehydrogenase, unlike lactate dehydrogenase, are well conserved during the evolutionary process.
Full text
PDF




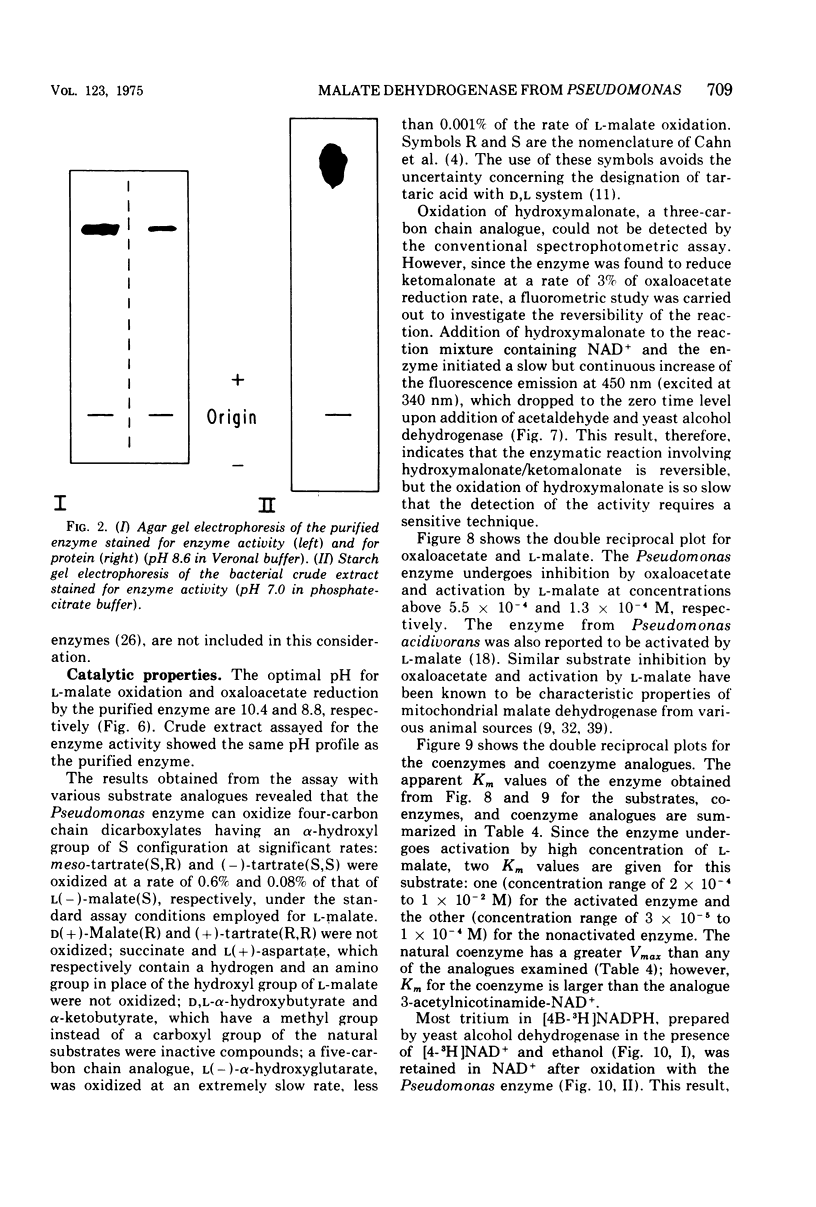
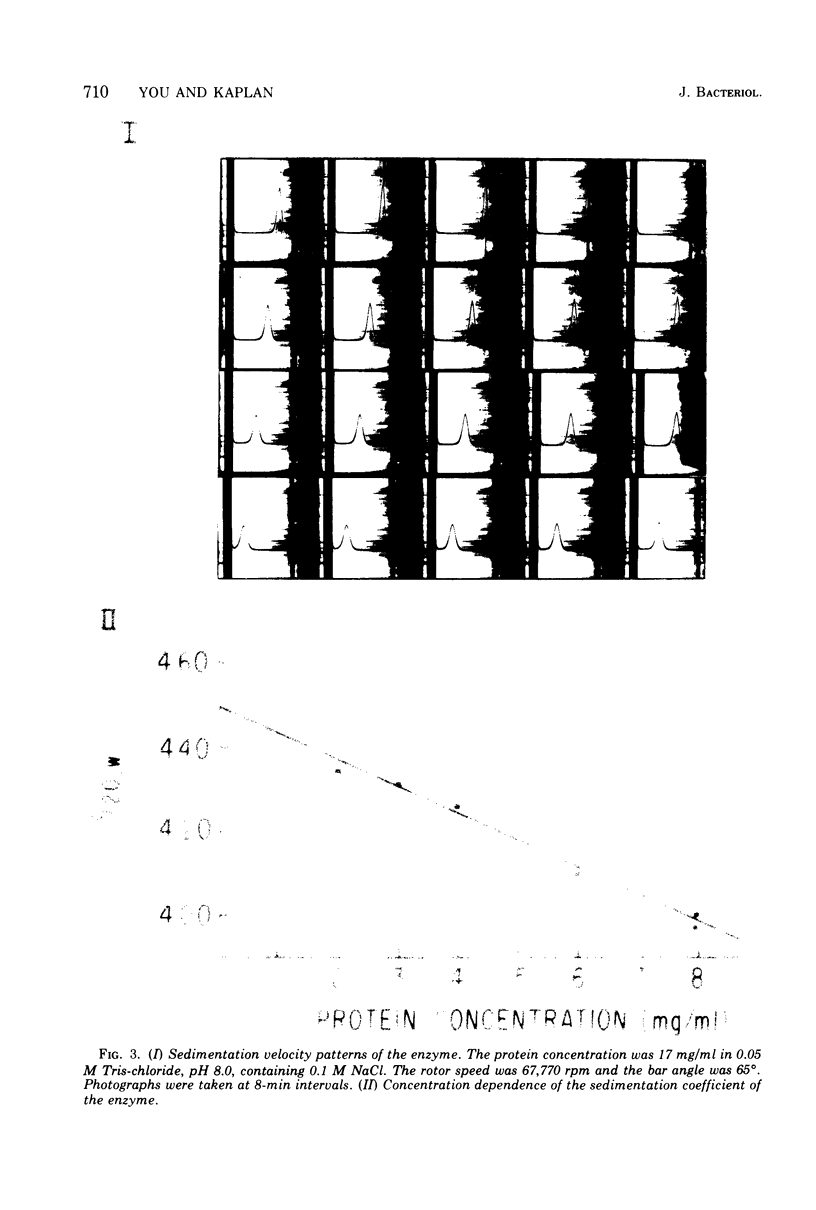
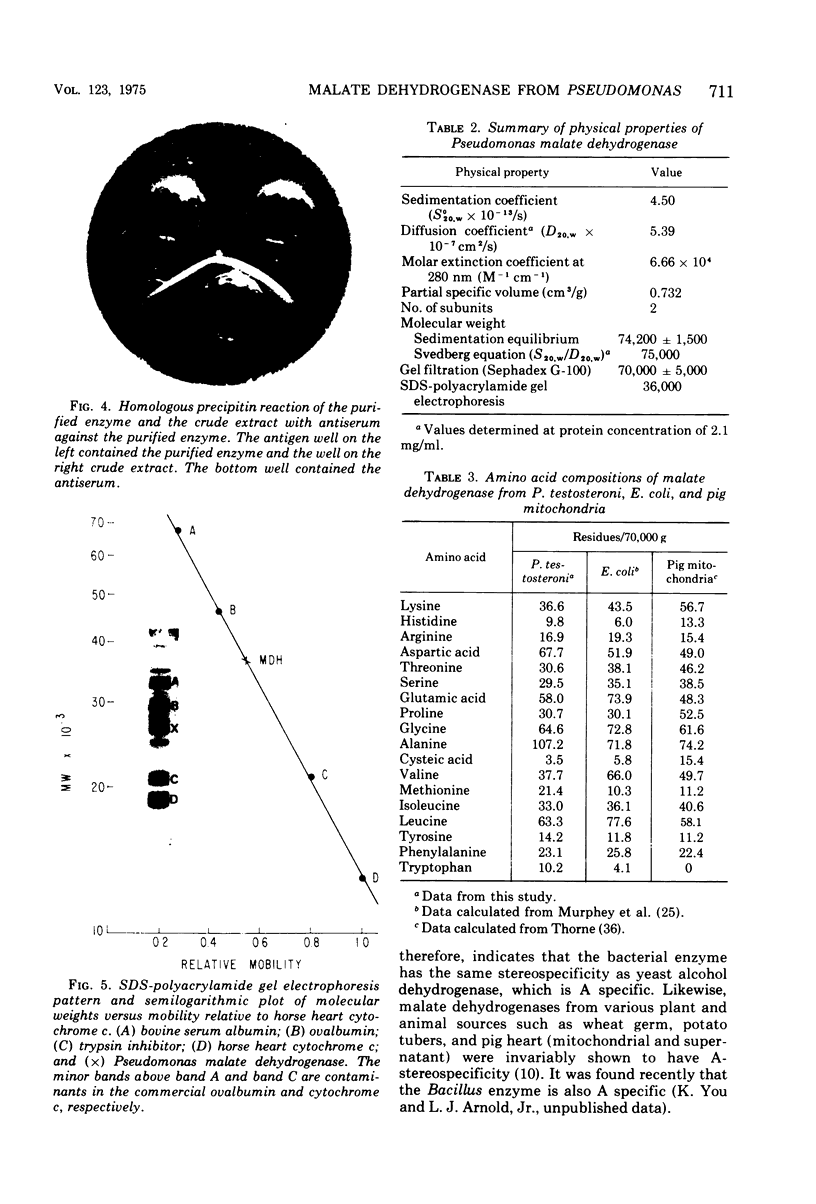


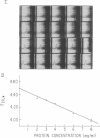
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison W. S., White H. B., 3rd, Connors M. J. Studies on the hydrogen-transfer reactions catalyzed by pyridine nucleotide linked dehydrogenases. Biochemistry. 1971 Jun 8;10(12):2290–2296. doi: 10.1021/bi00788a017. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- Cassman M., Englard S. Beef heart malic dehydrogenases. IV. Fluorescent binary and ternary complexes of the supernatant enzyme. J Biol Chem. 1966 Feb 25;241(4):787–792. [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- DAVIES D. D., KUN E. Isolation and properties of malic dehydrogenase from ox-heart mitochondria. Biochem J. 1957 Jun;66(2):307–316. doi: 10.1042/bj0660307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. D., Teixeira A., Kenworthy P. The stereospecificity of nicotinamide-adenine dinucleotide-dependent oxidoreductases from plants. Biochem J. 1972 Apr;127(2):335–343. doi: 10.1042/bj1270335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K., Wolfe R. G. Malic dehydrogenase. VI. A kinetic study of hydroxymalonate inhibition. J Biol Chem. 1968 Aug 10;243(15):4123–4130. [PubMed] [Google Scholar]
- Harada K., Wolfe R. G. Malic dehydrogenase. VII. The catalytic mechanism and possible role of identical protein subunits. J Biol Chem. 1968 Aug 10;243(15):4131–4137. [PubMed] [Google Scholar]
- Heyde E., Ainsworth S. Kinetic studies on the mechanism of the malate dehydrogenase reaction. J Biol Chem. 1968 May 10;243(9):2413–2423. [PubMed] [Google Scholar]
- KRASNA A. I. The inhibition of fumarase and malic dehydrogenase by DL-beta-fluoromalic acid. J Biol Chem. 1961 Mar;236:749–753. [PubMed] [Google Scholar]
- Kaplan N. O. Pyridine nucleotide transhydrogenases. Harvey Lect. 1971;66:105–133. [PubMed] [Google Scholar]
- Kitto G. B., Wassarman P. M., Kaplan N. O. Enzymatically active conformers of mitochondrial malate dehydrogenase. Proc Natl Acad Sci U S A. 1966 Aug;56(2):578–585. doi: 10.1073/pnas.56.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn L. D., Jakoby W. B. Tartaric acid metabolism. IV. Crystalline L-malic dehydrogenase from Pseudomonas acidovorans. J Biol Chem. 1968 May 25;243(10):2472–2478. [PubMed] [Google Scholar]
- Long G. L., Kaplan N. O. Diphosphopyridine nucleotide-linked D-lactate dehydrogenases from the horseshoe crab, Limulus polyphemus and the seaworm, Nereis virens. I. Physical and chemical properties. Arch Biochem Biophys. 1973 Feb;154(2):696–710. doi: 10.1016/0003-9861(73)90025-8. [DOI] [PubMed] [Google Scholar]
- MARCUS P. I., TALALAY P. Induction and purification of alpha- and beta-hydroxysteroid dehydrogenases. J Biol Chem. 1956 Feb;218(2):661–674. [PubMed] [Google Scholar]
- Murphey W. H., Barnaby C., Lin F. J., Kaplan N. O. Malate dehydrogenases. II. Purification and properties of Bacillus subtilis, Bacillus stearothermophilus, and Escherichia coli malate dehydrogenases. J Biol Chem. 1967 Apr 10;242(7):1548–1559. [PubMed] [Google Scholar]
- Murphey W. H., Kitto G. B., Everse J., Kaplan N. Malate dehydrogenases. I. A survey of molecular size measured by gel filtration. Biochemistry. 1967 Feb;6(2):603–610. doi: 10.1021/bi00854a031. [DOI] [PubMed] [Google Scholar]
- RAVAL D. N., WOLFE R. G. Malic dehydrogenase. II. Kinetic studies of the reaction mechanism. Biochemistry. 1962 Mar;1:263–269. doi: 10.1021/bi00908a012. [DOI] [PubMed] [Google Scholar]
- SIEGEL L., ENGLARD S. Beef-heart malic dehydrogenases. I. Properties of the enzyme purified from extracts of acetone-dried powders. Biochim Biophys Acta. 1961 Nov 25;54:67–76. doi: 10.1016/0006-3002(61)90938-6. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Sulebele G. Catalytic mechanism of pig heart mitochondrial malate dehydrogenase studied by kinetics at equilibrium. Biochemistry. 1969 Jun;8(6):2543–2550. doi: 10.1021/bi00834a042. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Sulebele G. Equilibrium kinetic study of the mechanism of mitochondrial and supernatant malate dehydrogenases of bovine heart. Biochim Biophys Acta. 1969;185(2):297–304. doi: 10.1016/0005-2744(69)90422-7. [DOI] [PubMed] [Google Scholar]
- THORNE C. J., KAPLAN N. O. Physicochemical properties of pig and horse heart mitochondrial malate dehydrogenase. J Biol Chem. 1963 May;238:1861–1868. [PubMed] [Google Scholar]
- THORNE C. J. Properties of mitochondrial malate dehydrogenases. Biochim Biophys Acta. 1962 Jun 4;59:624–633. doi: 10.1016/0006-3002(62)90642-x. [DOI] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Chemical characterization of D-lactate dehydrogenase from Escherichia coli B. J Biol Chem. 1968 May 25;243(10):2579–2586. [PubMed] [Google Scholar]
- WOLFE R. G., NEILANDS J. B. Some molecular and kinetic properties of heart malic dehydrogenase. J Biol Chem. 1956 Jul;221(1):61–69. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wolfenstein C., Englard S., Listowsky I. Bovine heart malic dehydrogenases. 8. Subunit structure and molecular weight of the supernatant enzyme. J Biol Chem. 1969 Dec 10;244(23):6415–6419. [PubMed] [Google Scholar]
- YOSHIDA A. PURIFICATION AND CHEMICAL CHARACTERIZATION OF MALATE DEHYDROGENASE OF BACILLUS SUBTILIS. J Biol Chem. 1965 Mar;240:1113–1117. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]