Abstract
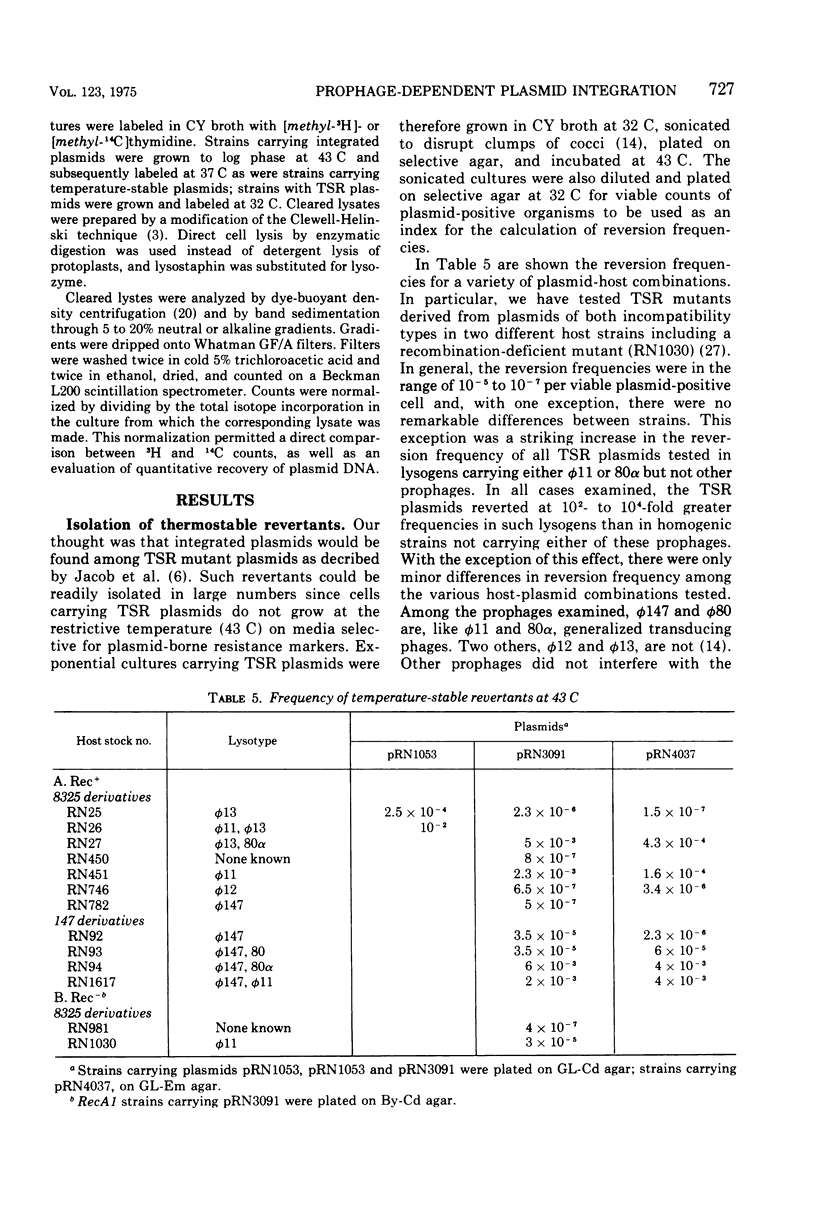
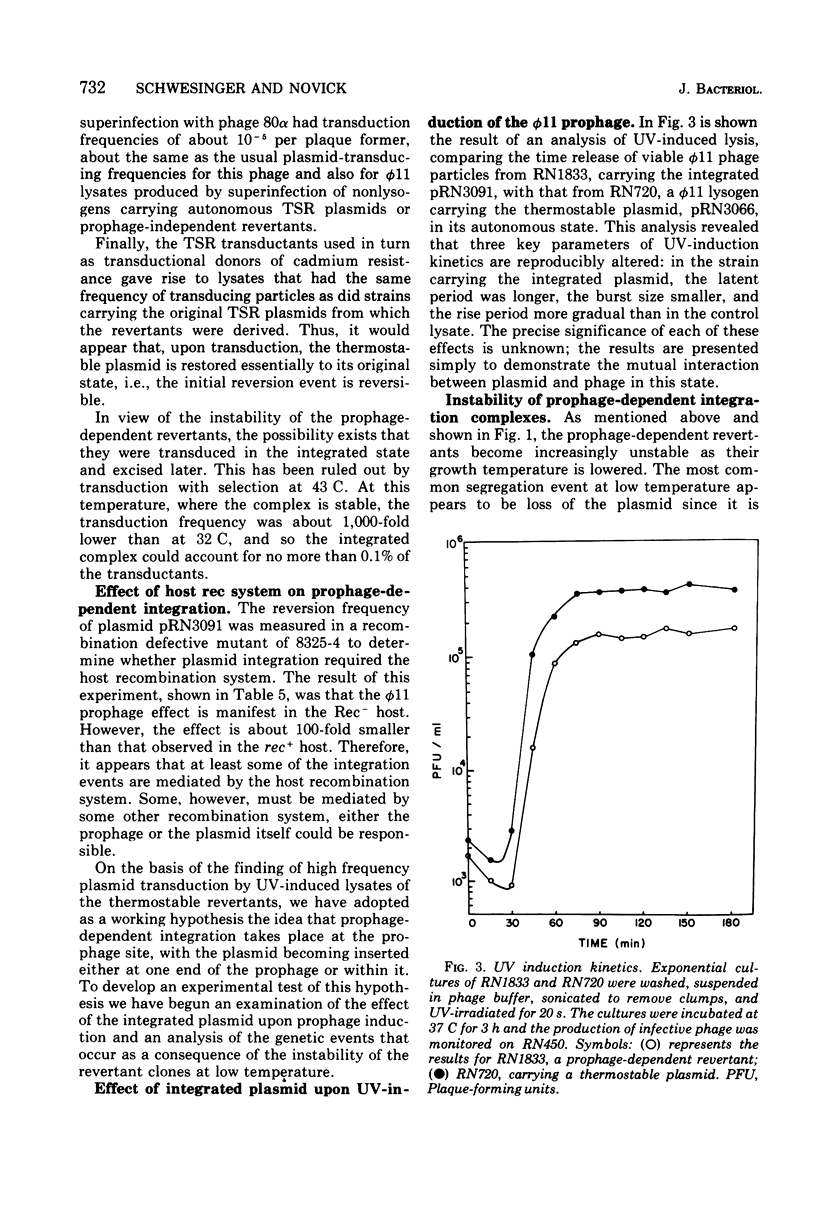
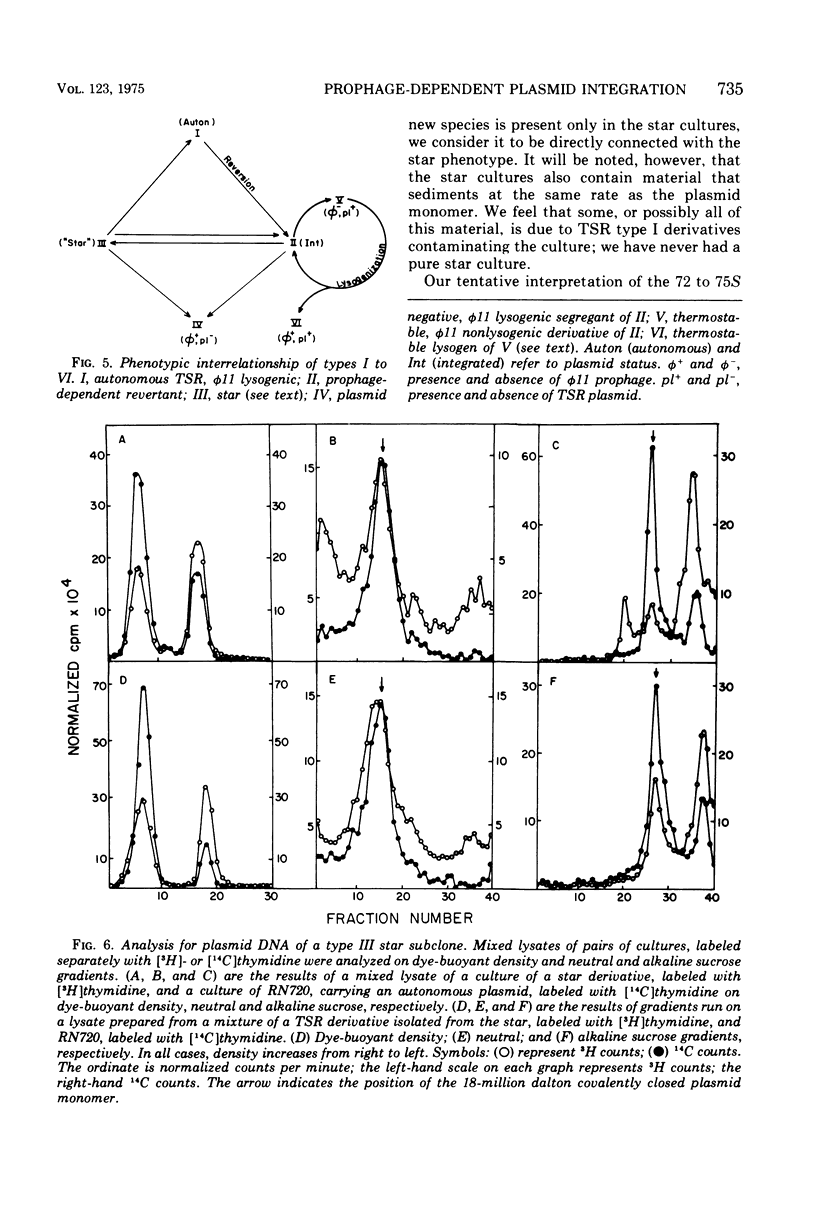
A study has been done of reversion to thermostability of thermosensitive, replication-defective (TSR) mutant penicillinase plasmids. All three of the expected classes of reversions were encountered: back mutation, suppression, and integration. The latter class was examined in some detail and it was found that the presence of the phi 11 phophage enhance the frequency of reversion by integration some 103-fold. Prophage-dependent integration resulted in inactivation of plasmid-linked arsenate and arsenite resistance; these revertant strains gave rise to high frequency tranducing lysates where the plasmid was restored upon transduction to its original TSR state including recovery of these resistances. The integrated plasmid-prophage complexes were stable at high temperatures (43 C) but slow growing and unstable at low (32 C); loss of either plasmid or prophage restored normal growth and stability. Sometimes restoration of the plasmid to its autonomous TSR state was observed and molecular studies showed that in most cases the plasmid was essentially the same size as before integration. In some cases an excision complex was recovered that was more than twice the size of the plasmid and could have been a plasmid-phage co-integrate. Integration also took place in the absence of the ł 11 prophage. These integrations retained all plasmid-linked resistances, were stable at all temperatures, and gave rise to low frequency transducing lysates in which the integrated state was retained upon transduction. On the basis of these results it is suggested that the prophage promotes integration at or near its attachment site.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G., SIX E. Inheritance of prophage P2 in bacterial crosses. Virology. 1958 Oct;6(2):357–381. doi: 10.1016/0042-6822(58)90089-8. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBNAU E., STOCKER B. A. GENETICS OF PLASMIDS IN SALMONELLA TYPHIMURIUM. Nature. 1964 Dec 12;204:1112–1113. doi: 10.1038/2041112a0. [DOI] [PubMed] [Google Scholar]
- Folkmanis A., Freifelder D. Studies on lysogeny in Escherichia coli with bacteriophage lambda. Physical observation of the insertion process. J Mol Biol. 1972 Mar 14;65(1):63–73. doi: 10.1016/0022-2836(72)90492-5. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J. Cell genetics and hereditary symbiosis. Physiol Rev. 1952 Oct;32(4):403–430. doi: 10.1152/physrev.1952.32.4.403. [DOI] [PubMed] [Google Scholar]
- Lindberg M., Novick R. P. Plasmid-specific transformation in Staphylococcus aureus. J Bacteriol. 1973 Jul;115(1):139–145. doi: 10.1128/jb.115.1.139-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., HASHIMOTO H., KONO M., MORIMURA M. DRUG RESISTANCE OF STAPHYLOCOCCI. II. JOINT ELIMINATION AND JOINT TRANSDUCTION OF THE DETERMINANTS OF PENICILLINASE PRODUCTION AND RESISTANCE TO MACROLIDE ANTIBIOTICS. J Bacteriol. 1965 Apr;89:988–992. doi: 10.1128/jb.89.4.988-992.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Brodsky R. Studies on plasmid replication. I. Plasmid incompatibility and establishment in Staphylococcus aureus. J Mol Biol. 1972 Jul 21;68(2):285–302. doi: 10.1016/0022-2836(72)90214-8. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Peyru G., Wexler L. F., Novick R. P. Naturally occurring penicillinase plasmids in Staphylococcus aureus. J Bacteriol. 1969 Apr;98(1):215–221. doi: 10.1128/jb.98.1.215-221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. DOMINANCE OF THE INDUCIBLE STATE IN STRAINS OF STAPHYLOCOCCUS AUREUS CONTAINING TWO DISTINCT PENICILLINASE PLASMIDS. J Bacteriol. 1965 Aug;90:370–374. doi: 10.1128/jb.90.2.370-374.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedler H., Heiss B. Multiple copies of the insertion-DNA sequences IS1 and IS2 in the chromosome of E. coli K-12. Mol Gen Genet. 1973 May 9;122(3):267–277. doi: 10.1007/BF00278602. [DOI] [PubMed] [Google Scholar]
- Signer E. R. Lysogeny: the integration problem. Annu Rev Microbiol. 1968;22:451–488. doi: 10.1146/annurev.mi.22.100168.002315. [DOI] [PubMed] [Google Scholar]
- Smith K., Novick R. P. Genetic studies on plasmid-linked cadmium resistance in Staphylococcus aureus. J Bacteriol. 1972 Nov;112(2):761–772. doi: 10.1128/jb.112.2.761-772.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starlinger P., Saedler H. Insertion mutations in microorganisms. Biochimie. 1972;54(2):177–185. doi: 10.1016/s0300-9084(72)80102-0. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman L., Goering R. V., Novick R. P. Genetic control of chromosomal and plasmid recombination in Staphylococcus aureus. Genetics. 1974 Apr;76(4):681–702. doi: 10.1093/genetics/76.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman L., Novick R. P. Studies on plasmid replication. IV. Complementation of replication-defective mutants by an incompatibility-deficient plasmid. Mol Gen Genet. 1974;135(2):149–161. doi: 10.1007/BF00264782. [DOI] [PubMed] [Google Scholar]