Abstract
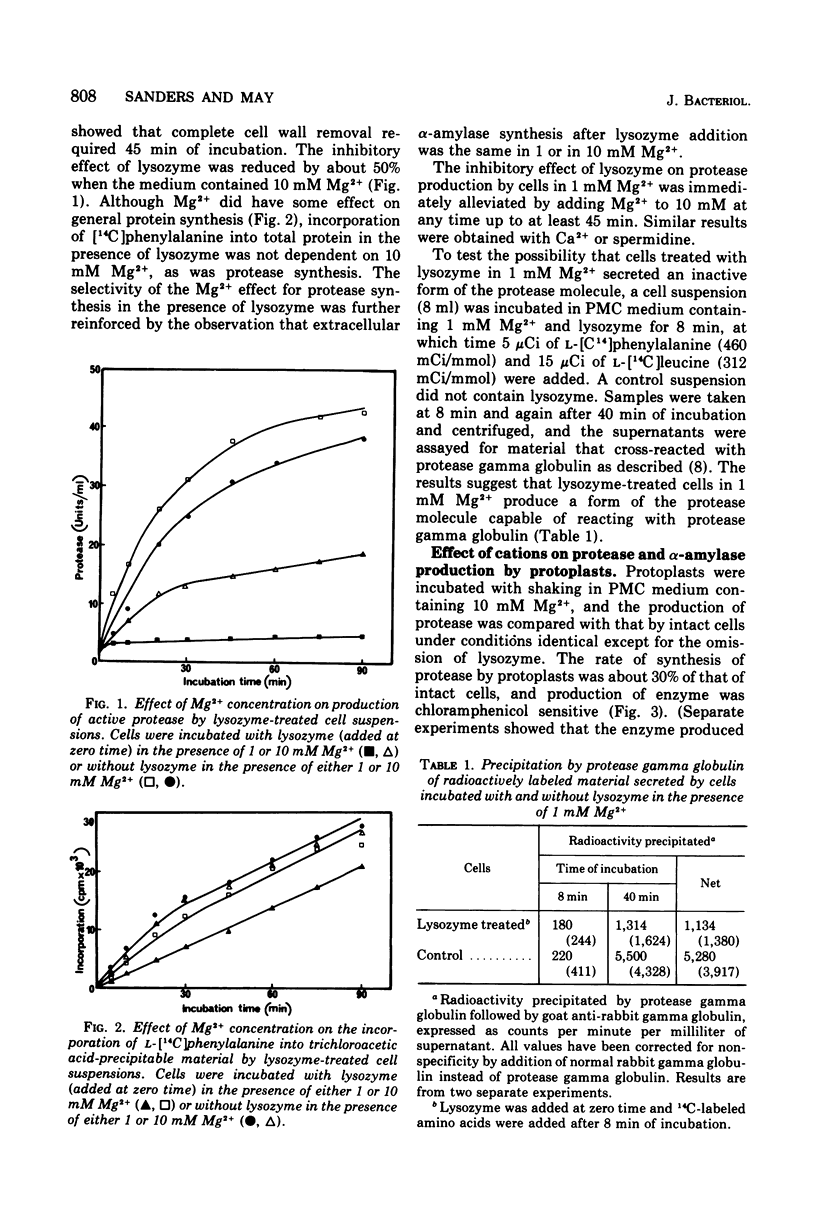
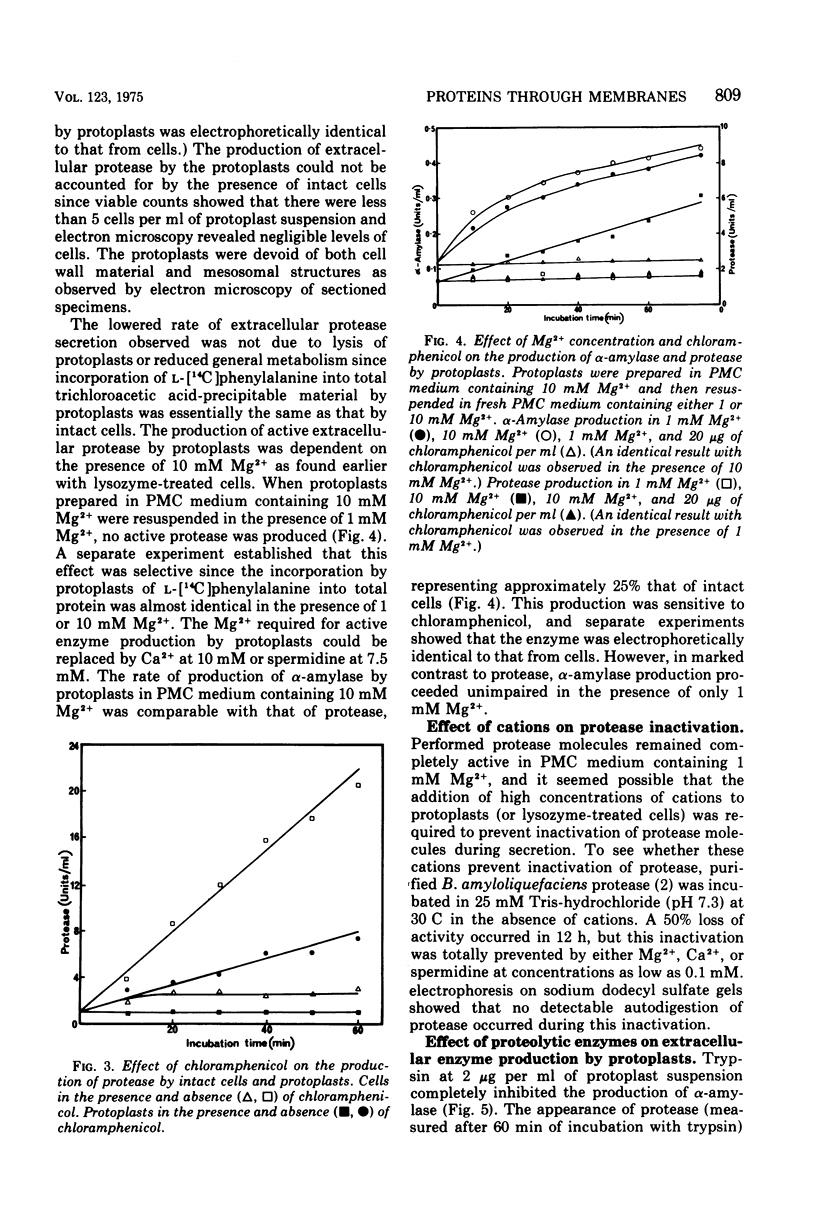
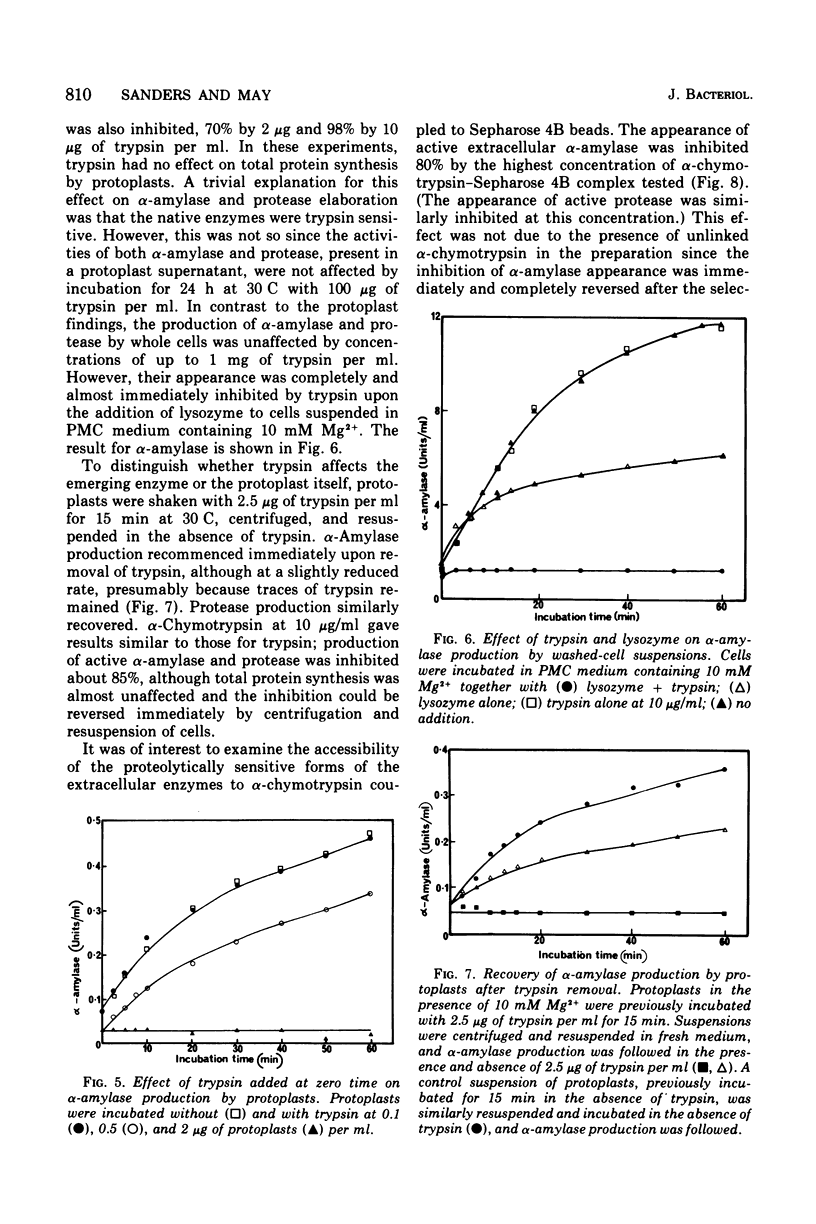
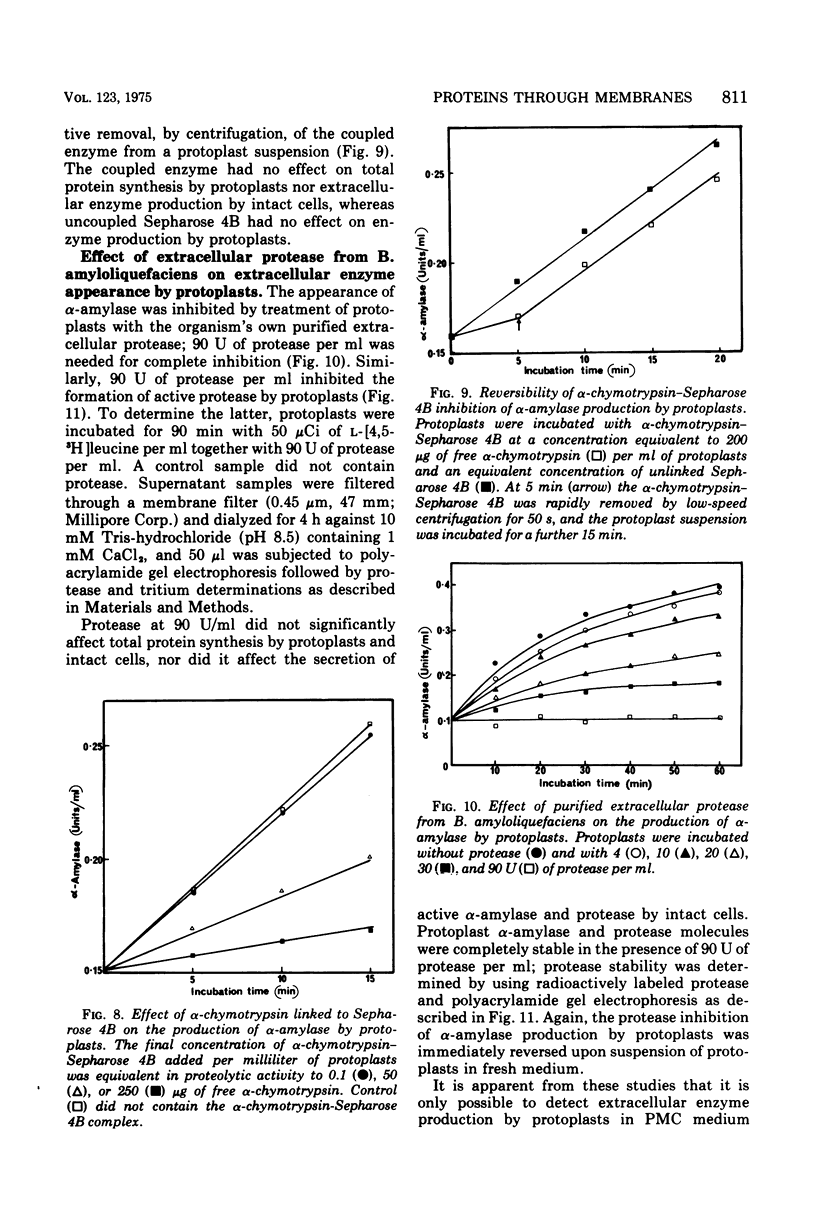
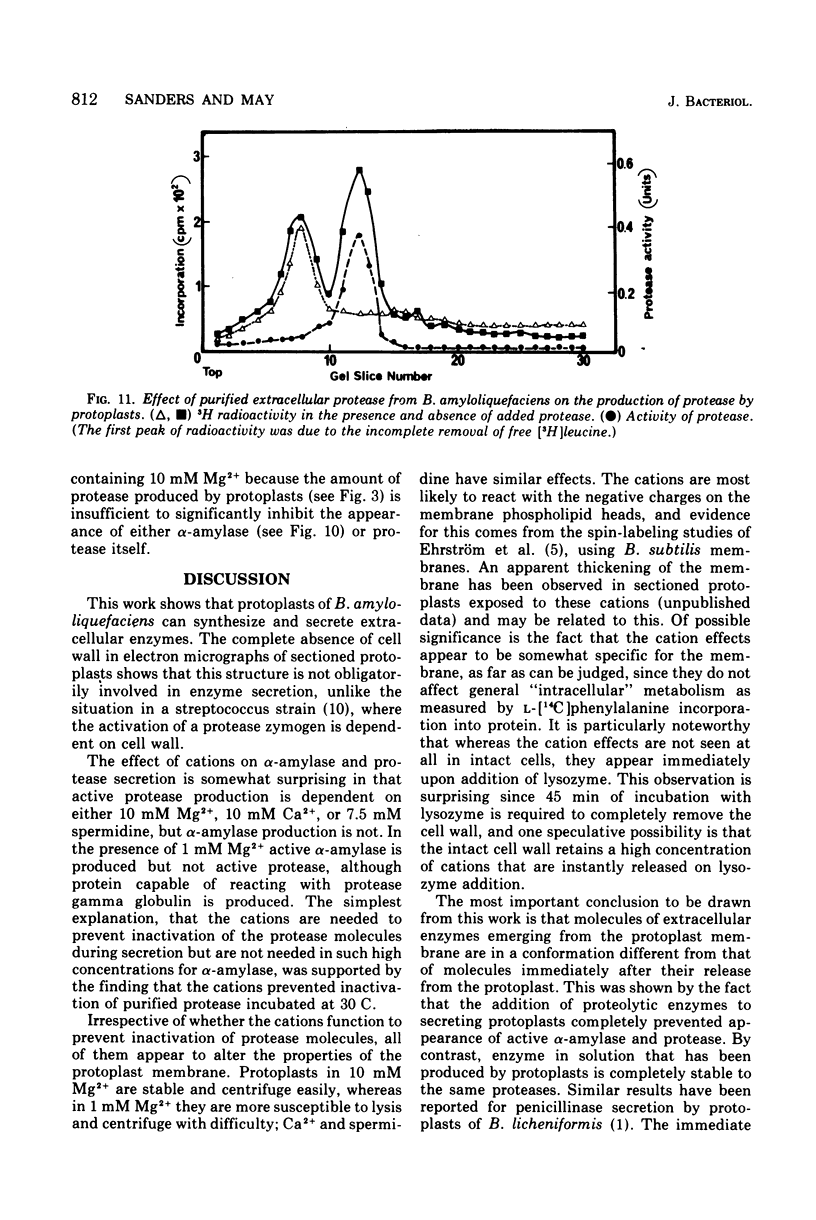
The production of extracellular alpha-amylase and protease by protoplasts of Bacillus amyloliquefaciens has been achieved. The production of enzymically active protease was totally dependent on a high concentration of either Mg2+, Ca2+, or spermidine, but production of active alpha-amylase was not. This cation dependence of protease production was seen immediately upon addition of lysozyme to intact cells. The cations could prevent the inactivation of protease and alter the cytoplasmic membrane configuration of protoplasts. Production of active alpha-amylase and protease by protoplasts was totally inhibited by proteolytic enzymes such as trypsin, alpha-chymotrypsin, or the organism's purified extracellular protease. The evidence suggests that these degradative enzymes act specifically on the emerging polypeptide of the extracellular enzyme and that the polypeptide emerges in a conformation different from that of the native molecule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettinger G. E., Lampen J. O. Evidence for the extrusion of an incompletely folded form of penicillinase during secretion by protoplasts of Bacillus licheniformis 749-C. Biochem Biophys Res Commun. 1971 Apr 2;43(1):200–206. doi: 10.1016/s0006-291x(71)80107-9. [DOI] [PubMed] [Google Scholar]
- Both G. W., McInnes J. L., Hanlon J. E., May B. K., Elliott W. H. Evidence for an accumulation of messenger RNA specific for extracellular protease and its relevance to the mechanism of enzyme secretion in bacteria. J Mol Biol. 1972 Jun 20;67(2):199–217. doi: 10.1016/0022-2836(72)90236-7. [DOI] [PubMed] [Google Scholar]
- Braatz J. A., Heath E. C. The role of polysaccharide in the secretion of protein by Micrococcus sodonensis. J Biol Chem. 1974 Apr 25;249(8):2536–2547. [PubMed] [Google Scholar]
- COLEMAN G., ELLIOTT W. H. Studies on alpha-amylase formation by Bacillus subtilis. Biochem J. 1962 May;83:256–263. doi: 10.1042/bj0830256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrström M., Eriksson L. E., Israelachvili J., Ehrenberg A. The effects of some cations and anions on spin labeled cytoplasmic membranes of Bacillus subtilis. Biochem Biophys Res Commun. 1973 Nov 16;55(2):396–402. doi: 10.1016/0006-291x(73)91100-5. [DOI] [PubMed] [Google Scholar]
- Gould A. R., May B. K., Elliott W. H. Accumulation of messenger RNA for extracellular enzymes as a general phenomenon in Bacillus amyloiquefaciens. J Mol Biol. 1973 Jan 10;73(2):213–219. doi: 10.1016/0022-2836(73)90324-0. [DOI] [PubMed] [Google Scholar]
- Gould A. R., May B. K., Elliott W. H. Release of extracellular enzymes from Bacillus amyloliquefaciens. J Bacteriol. 1975 Apr;122(1):34–40. doi: 10.1128/jb.122.1.34-40.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley R. W., Smeaton J. R. On the reaction between the extracellular ribonuclease of Bacillus amyloliquefaciens (barnase) and its intracellular inhibitor (barstar). J Biol Chem. 1973 Aug 25;248(16):5624–5626. [PubMed] [Google Scholar]
- LIU T. Y., ELLIOTT S. D. ACTIVATION OF STREPTOCOCCAL PROTEINASE AND ITS ZYMOGEN BY BACTERIAL CELL WALLS. Nature. 1965 Apr 3;206:33–34. doi: 10.1038/206033a0. [DOI] [PubMed] [Google Scholar]
- May B. K., Elliott W. H. Characteristics of extracellular protease formation by Bacillus subtilis and its control by amino acid repression. Biochim Biophys Acta. 1968 May 21;157(3):607–615. doi: 10.1016/0005-2787(68)90158-5. [DOI] [PubMed] [Google Scholar]
- May B. K., Elliott W. H. Selective inhibition of extracellular enzyme synthesis by removal of cell wall from Bacillus subtilis. Biochim Biophys Acta. 1968 Sep 24;166(2):532–537. doi: 10.1016/0005-2787(68)90240-2. [DOI] [PubMed] [Google Scholar]
- May B. K., Elliott W. H. Synthesis and properties of a protoplast-bursting factor from Bacillus amyloliquefaciens. Biochem Biophys Res Commun. 1970 Oct 9;41(1):199–205. doi: 10.1016/0006-291x(70)90488-2. [DOI] [PubMed] [Google Scholar]
- Redman C. M., Sabatini D. D. Vectorial discharge of peptides released by puromycin from attached ribosomes. Proc Natl Acad Sci U S A. 1966 Aug;56(2):608–615. doi: 10.1073/pnas.56.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Silverman P., Haverback B. J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- Smeaton J. R., Elliott W. H. Isolation and properties of a specific bacterial ribonuclease inhibitor. Biochim Biophys Acta. 1967;145(3):547–560. doi: 10.1016/0005-2787(67)90115-3. [DOI] [PubMed] [Google Scholar]


