Abstract
Perforant path long-term potentiation (LTP) in intact mouse hippocampal dentate gyrus increased the neuron-specific, growth-associated protein GAP-43 mRNA in hilar cells 3 days after tetanus, but surprisingly not in granule cells, the perforant path target. This increase was positively correlated with level of enhancement and restricted to central hilar cells on the side of stimulation. Blockade of LTP by puffing dl-aminophosphonovalerate (APV), an N-methyl-d-aspartate (NMDA) receptor blocker into the molecular layer, eliminated LTP-induced GAP-43 mRNA elevation in hilar cells. To determine whether the mRNA elevation was mediated by transcription, LTP was studied in transgenic mice bearing a GAP-43 promoter-lacZ reporter gene. Promoter activity as indexed by Transgene expression (PATE) increased as indicated by blue staining of the lacZ gene product, β-galactosidase. Potentiation induced a blue band bilaterally in the inner molecular layer of the dentate gyrus along the entire septotemporal axis. Because mossy cells are the only neurons in the central hilar zone that project to the inner molecular layer bilaterally along the entire septotemporal axis and LTP-induced activation of PATE in this zone was confined to the side of stimulation, we concluded that mossy cells were unilaterally activated, increasing synthesis of β-galactosidase, which was transported bilaterally. Neither granule cells nor pyramidal cells demonstrated increased PATE or increased GAP-43 mRNA levels. These results and recent evidence indicating the necessity of hilar neurons for LTP point to previously unheralded mossy cells as potentially critical for perforant path LTP and the GAP-43 in these cells as important for LTP persistence lasting days.
Keywords: synaptic plasticity, gene expression, transgenic mouse, transcription, memory storage
While it is now well established that long-term potentiation (LTP), a physiological model of the memory storage process, activates immediate early genes encoding transcription factors (1, 2), there is no evidence to our knowledge indicating what target genes are activated by these transcription factors. GAP-43,¶ the presynaptically localized, growth-associated protein whose expression is up-regulated during axonal outgrowth in development and axonal regeneration (3), and whose phosphorylation is increased after LTP (4), is one likely candidate target gene (see also ref. 5). A previous study of chronic recording in intact freely moving rats 3 days after LTP induction revealed that perforant path LTP altered GAP-43 mRNA expression (6). To explore the hypothesis that the alteration in GAP-43 mRNA was related to altered transcriptional activity of the GAP-43 gene in hippocampal neurons after LTP, we first studied the effect of LTP in the intact mouse on the endogenous pattern of GAP-43 mRNA transcript levels with in situ hybridization. We then studied the effect of LTP on promoter activation in transgenic mice bearing a GAP-43 promoter-lacZ reporter construct.
MATERIALS AND METHODS
Animals.
Mouse strain C57BL/6 was obtained from The Jackson Laboratory. Heterozygous GAP-43/lacZ transgenic mouse line 252 bearing 6-kb 5′-flanking and 11-kb first intron sequence driving lacZ reporter gene was bred with C57BL/6 mice, and homozygous transgenic mice were initially screened by genomic DNA Southern or dot hybridization. Constitutive expression of the transgene in hippocampus in GAP-43/lacZ transgenic mouse line 252 has been described (7, 8). Thirty-five male homozygous transgenic mice (2 months old) were used in the electrophysiological and histochemical analysis.
Electrophysiology.
In vivo LTP in anesthetized mice was performed essentially as described previously (9). Potentiated responses were measured by percent baseline population spike amplitude. Mean potentiation of combined 1-, 2-, and 3-day LTP group animals was 255% ± 40.1% (n = 20; mean ± SEM) over the 2-hr recording session and did not show any significant difference from that of wild-type C57BL/6 mice (229.4% ± 36.3%, P > 0.10). Mean response of the low-frequency control (LFC) animals during the 2-hr recording period was 101.1 ± 5.5 (n = 15). In the LTP experiment with dl-aminophosphonovalerate (APV; Sigma) ejection, 80 nl (1.6 nmol) of APV dissolved in 20 mM Tris⋅HCl (pH 8.0) was pressure-ejected from the recording micropipette into the dentate gyrus molecular layer, using procedures described earlier (10). APV was delivered 15 min prior to high-frequency tetanic stimulation. Equivalent volumes of 20 mM Tris vehicle were ejected into the same region of the hippocampus in control group animals. Potentiated responses in the molecular layer were recorded for 60 min and the baseline field excitatory postsynaptic potential (fEPSP) was analyzed. After in vivo recording, animals were placed on a heating pad overnight and allowed to recover from anesthesia.
After using this “acute-then-chronic” procedure, recovered mice were reanesthetized 1–3 days after LTP induction and perfused transcardially with 2% paraformaldehyde in 0.1 M Pipes buffer (pH 6.9) in 2 mM MgCl2 and 2 mM EGTA. Brain tissue was postfixed in perfusate for 1 hr and kept overnight in 30% sucrose in 1× phosphate-buffered saline (PBS) with 2 mM MgCl2. Two or more sets of coronal brain sections of 20 μm were collected from each animal for the analysis of GAP-43 mRNA by in situ hybridization and promoter activation by 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) staining of β-galactosidase, the lacZ gene product. At the end of the electrophysiological recording, animals were coded so that for the quantitative analysis of mRNA and X-Gal staining, the experiment was blind as to condition.
In Situ Hybridization.
In situ hybridization using 35S-labeled antisense riboprobe complementary to rat GAP-43 cDNA and quantitative analysis for mRNA expression in the granule cells, CA3, and CA1 cell region were performed as described (11). Because the autoradiographic image in the hilar region showed that GAP-43 mRNA expression was in patches overlying individual cells, quantitation in the hilar region was performed as follows: silver grain density within the circular template of 25-μm diameter was measured in each patch and summated for individual sections. Mean value of areal grain density measured for each animal was obtained by measuring grain density within circle templates of eight to ten sections. The comparison of GAP-43 mRNA levels among ipsi- and contralateral sides of LTP, LFC, and unoperated group animals in hilar cells, granule cells, and CA1 and CA3 pyramidal cells was performed separately for the septal and the temporal hippocampus. One-way ANOVA with Fisher’s predicted least-square difference (PLSD) test was used for statistical comparison (12).
X-Gal Staining.
Sections were treated for 15 min first with 1× PBS with 2 mM MgCl2 and then with 1× PBS with 2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% Nonidet P-40 (Sigma). X-Gal reagent (Boehringer Mannheim) was dissolved in dimethyl sulfoxide and added in a final concentration of 1 mg/ml in the staining solution consisting of 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2, 0.01% sodium deoxycholate, and 0.02% Nonidet P-40 in 1× PBS. Slides were stained for 16 hr in the dark at 37°C, counterstained with neutral red, dehydrated in ethanol, and rinsed in clearing agent (Lipshaw, Fisher) before coverslipping. Quantitative analysis of X-Gal staining in the central hilar region (CHR) or the infragranular hilar region (IHR) of the hilus was determined by manually counting the number of X-Gal-stained hilar cells under a 10× objective. Hilar cells whose X-Gal-stained areas were 150 μm2 and 25 μm2 or larger in CHR and IHR, respectively, were counted for quantitation. Percent X-Gal-stained cells in the ipsilateral side compared with contralateral side was then determined after averaging eight to ten sections per animal over the septal or temporal hippocampus. Statistical comparison of X-Gal staining among ipsi- and contralateral sides of LTP group and LFC group animals was performed by one-way ANOVA with Fisher’s PLSD test.
RESULTS
In our earlier report on LTP, which used chronic recording in albino rats (6), we observed a significant decrease in the GAP-43 mRNA in CA3 cells 3 days after perforant path LTP. In the present “acute-then-chronic” procedure in mice, GAP-43 mRNA in CA3 pyramidal cells was also significantly decreased 3 days after LTP induction, comparing the ipsilateral side versus the contralateral side (P < 0.05; n = 5). Also consistent with this earlier report was the absence of a detectable change in GAP-43 mRNA expression in granule cells or in CA1 pyramidal cells at any time, as well as the lack of alteration of mRNA expression in CA3 cells 1 and 2 days after LTP (n = 5 at each day). Therefore, the present “acute-then-chronic” paradigm in mouse yielded results that paralleled those observed in the chronic conscious rat (6).
We discovered that 48 hr after the tetanus, GAP-43 mRNA expression in mouse dentate hilar cells was significantly elevated. However, comparison in the entire zone 4 (13) of mRNA levels encompassing the different cell types did not show any difference between ipsi- and contralateral sides in 1-, 2-, and 3-day LTP group animals (data not shown). Because inhibitory neurons are predominantly localized to the IHR (see Fig. 1A) (13, 14), we subdivided zone 4 of the hilus into the CHR, where glutamate-containing mossy cells are present, and the IHR, where γ-aminobutyrate (GABA)-containing cells are present.
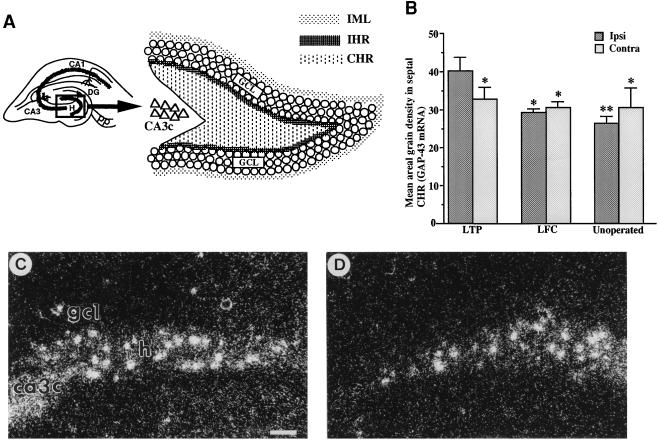
Figure 1.
(A) Schematic diagram of dentate gyrus with Inset showing location of major hilar zones. Delineation of the inner molecular layer (IML), IHR, and CHR of dentate gyrus. GCL, granule cell layer; H, hilus; DG, dentate gyrus; CA3c, CA3, and CA1 are pyramidal cell fields; pp, perforant path. (B) Quantitative analysis of GAP-43 mRNA in septal CHR of the hippocampus of combined 2- and 3-day LTP (n = 10), 2- and 3-day LFC (n = 6), and unoperated control groups (n = 5). Levels of mRNA were compared between ipsi- and contralateral sides in septal CHR. The ipsilateral side of the LTP group showed a significant increase of mRNA levels compared with its contralateral side and to either side of LFC or unoperated control groups. Error bars represent SEM. ∗, P < 0.05; ∗∗, P < 0.01; one-way ANOVA. (C and D) Representative examples of the elevation of mRNA expression in hippocampal hilar region in LTP-induced animals. Coronal brain sections collected 3 days after LTP induction were used for in situ hybridization. Mean areal grain density in the ipsilateral side of CHR (C) was higher than that in the contralateral side (D). Note that the ipsilateral side (C) contains a higher number of silver grain “patches” than the contralateral side (D). Thus, the mean number of “patches” for 2- and 3-day LTP group animals was 19.2 ± 3.2 for ipsilateral side and 16.1 ± 1.9 for contralateral side. No alteration in expression was observed in granule cells. (Scale bar = 100 μm.)
In the 1 day post-LTP group (n = 5), no differences were found in hilar mRNA expression between ipsi- and contralateral sides in either CHR or IHR in either septal or temporal hippocampus. Indeed, the levels of mRNA expression were similar to those of the 1 day post-LFC group (n = 3). However, at 2 and 3 days after LTP induction (see Table 1), the ipsilateral side of septal CHR showed higher expression of GAP-43 mRNA compared with (i) the contralateral side (P < 0.05, one-way ANOVA; n = 10, 2 and 3 days combined), (ii) the ipsi- and contralateral sides of LFC group (n = 6; P < 0.05 for both sides), and (iii) the ipsi- and contralateral sides of an unoperated control group (n = 5, P < 0.01, P < 0.05, respectively; Fig. 1B). A representative example of an increase in GAP-43 mRNA in the ipsilateral side compared with contralateral side is shown in Fig. 1 C and D. The contralateral side in the LTP group showed no significant elevation of mRNA expression relative to the LFC group (P > 0.15 and P > 0.25 for ipsi- and contralateral sides, respectively, one-way ANOVA) or the unoperated group (P > 0.08 and P > 0.30 for ipsi- and contralateral sides, respectively). This indicates that LTP effects on GAP-43 gene expression are likely to represent an increase in level restricted to the cells of the ipsilateral hilus. No difference was found between ipsi- and contralateral sides in LFC and unoperated groups. In septal IHR, no significant difference was observed among ipsi- and contralateral sides of LTP, LFC, and unoperated control groups. No significant differences were observed in temporal CHR or IHR. Mean areal grain density in individual CHR groups is summarized in Table 1.
Since hilar mossy cells receive a major excitatory synaptic input from granule cells (15), the LTP-induced increase of GAP-43 mRNA in hilar cells might be related to the potentiated population spike observed in granule cells. Indeed, the increased GAP-43 mRNA expression in septal CHR was positively correlated with the mean population spike response (r = +0.72, P < 0.05, n = 10) in the 2- and 3-day LTP group animals. This suggests that the enhanced response in granule cells is translated with some fidelity into an elevation of GAP-43 gene expression in hilar cells.
Having discovered here an elevation of GAP-43 mRNA in hilar cells 2–3 days after tetanus in the initially anesthetized mouse, we analyzed GAP-43 mRNA levels after LTP in septal CHR in our previous study of chronically prepared rats (6). As observed in mouse, expression level of rat GAP-43 mRNA in the ipsilateral CHR region was 47% higher than contralateral side (P < 0.05; t test; n = 4) 3 days after LTP induction. This demonstrates that hilar cell GAP-43 mRNA elevation after LTP can be observed in the intact preparation of either rat or mouse. The fact that LTP produces correlated increases in GAP-43 gene expression in hilar cells while producing correlated decreases in expression of the same gene in CA3 pyramidal cells indicates that LTP sets into motion opposite adjustments in gene expression in different parts of the hippocampal network in response to the same synaptic enhancement.
We next determined whether elevation of mRNA expression in hilar cells requires LTP induction in granule cells. After APV, an N-methyl-d-aspartate (NMDA) receptor blocker, was puffed into the dentate molecular layer (n = 5), mean potentiation was 108.4% of baseline excitatory postsynaptic potential slope 1 hr after LTP induction, while the Tris-vehicle-treated group (n = 4) maintained a response of 180%. Three days after LTP, GAP-43 mRNA levels in the ipsilateral side as a percent of contralateral were 120.4% in Tris-vehicle group, whereas those in the APV-treated group were 99.0% (P < 0.05, Student t test). This result suggests that elevation of GAP-43 mRNA levels in hilar cells is a consequence of LTP induction mediated by activation of the NMDA receptor at hippocampal synapses.
The increase in GAP-43 mRNA may be due to increased transcriptional activity, which is here assessed by using GAP-43 promoter-lacZ transgenic mice (7) to investigate GAP-43 promoter activity as indexed by transgene expression (PATE), which was previously characterized in adults (8) and over the course of development (16). To our initial surprise, LTP induced a band of X-Gal staining of β-galactosidase in the dentate gyrus restricted to the inner molecular layer. The blue band was present bilaterally throughout the septotemporal axis of the hippocampus (Fig. 2 A and B) compared with LFC or unoperated animals (Fig. 2 C and D). Because granule cells activate hippocampal mossy cells in the dentate hilus (15), septal mossy cells project bilaterally to the inner molecular layer in the dentate gyrus (17, 18) and mossy cells innervate granule cells (36). We presumed that LTP of the perforant path activated mossy cells, unilaterally increasing GAP-43 PATE (Fig. 3B). The increase in β-galactosidase protein in mossy cells on the side of stimulation would then be axonally transported to mossy cell targets bilaterally.
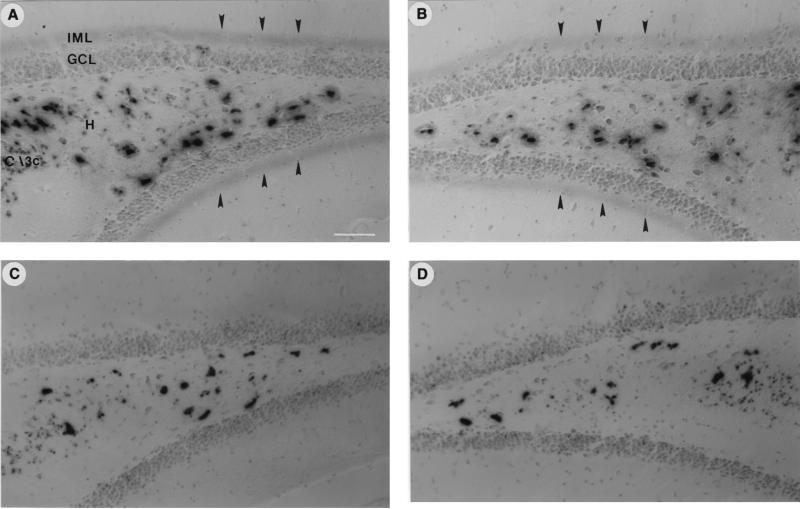
Figure 2.
Activation by LTP of GAP-43 promoter activity in dentate gyrus of GAP-43/lacZ transgenic mouse. (A and B) Arrowheads point to LTP-induced X-Gal staining of β-galactosidase activity in the inner molecular layer (IML) just external to the hippocampal granule cell layer (GCL) in transgenic mice. (C and D) Lack of induction of PATE in IML in LFC animals. Note greater number of X-Gal-stained cells (P < 0.05) were observed in the ipsilateral hilus (H) of the LTP group (A), compared with the contralateral side (B) and LFC animals (C and D). No X-Gal staining was observed in the granule cell layer (GCL) or their axons, the mossy fibers in either the LTP or the LFC group. Note that in the central hilar region, the ipsilateral side of the LTP group showed stronger staining intensity as well as increased numbers of stained cells compared with the contralateral side or either side of the LFC group. (Scale bar in A = 50 μm.)
Figure 3.
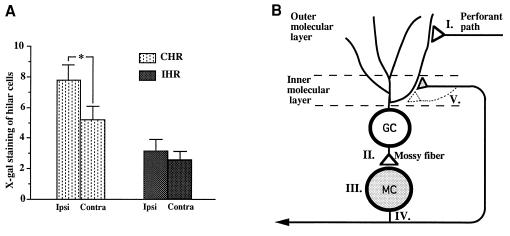
(A) Mean number of X-Gal-stained cells in the ipsilateral side of the 2- and 3-day LTP group animals with GAP-43 PATE in the inner molecular layer (IML; n = 7) in CHR or in IHR compared with the number of stained cells on the contralateral side. Error bars represent SEM. ∗, P < 0.05; one-way ANOVA. In the group sacrificed 1 day after LTP induction, 1 of 5 animals showed transgene induction in the IML. In animals sacrificed 2 and 3 days after LTP induction, 7 of 10 showed β-galactosidase in the inner molecular layer, whereas only 1 of 12 LFC and unoperated animals showed expression in that same region (P < 0.05; χ2 analysis). (B) Hypothetical scheme to indicate (i) the origin of the X-Gal-staining band induced by LTP in the inner molecular layer bilaterally and (ii) potential growth of mossy cell (MC) axon terminals. Two to three days after LTP induction, GAP-43 PATE in mossy cells leads to bilateral axonal transport of β-galactosidase. Specifically, tetanic stimulation of perforant path (I) induces potentiated responses in granule cells (GC). After receiving excitatory synaptic input from granule cells (II), transgene and endogenous GAP-43 mRNA expression (III) is induced in MC. β-Galactosidase induced in MC is transported bilaterally into the axonal terminal region in the inner molecular layer (IV). Arborization of axonal terminals (V) after LTP increases GAP-43 expression in the mossy cells is proposed.
These considerations predict that LTP should increase β-galactosidase in mossy cells restricted to the side of stimulation. We analyzed X-Gal staining in CHR, where mossy cells are found, or in IHR, where basket cells are found (Fig. 3A), by counting X-Gal-stained cells (see Materials and Methods). When the 2- and 3-day LTP group animals showing X-Gal staining in the inner molecular layer were combined, the mean number of X-Gal-stained cells in septal CHR was significantly higher in the ipsilateral side than the contralateral side (P < 0.05; one-way ANOVA; n = 7), whereas in IHR X-Gal staining showed no difference between ipsi- and contralateral sides (Fig. 3A). The increase in PATE on the ipsilateral side of CHR (54% compared with contralateral) was greater than that seen with GAP-43 mRNA (22% compared with contralateral). Differences that exist between the two in the 5′ recognition elements required and in posttranscriptional processing could contribute to these observed differences. In CHR, no difference in number of X-Gal-stained cells was found between the contralateral side of the LTP group and either ipsi- or contralateral sides of the LFC group (n = 6), suggesting that GAP-43 PATE in cells in the contralateral hilus is not influenced by LTP. No significant alteration of number of X-Gal-stained cells was found in the hilar region of temporal hippocampus (P > 0.70 in CHR, P > 0.85 in IHR; one-way ANOVA; n = 7), suggesting that the induction of GAP-43 gene is restricted to septal CHR, the target site for perforant path fibers activated by our stimulation (19). LTP induction of X-Gal staining of β-galactosidase was cell selective, as neither granule cells nor CA3 or CA1 cells demonstrated detectable alterations.
DISCUSSION
The increased expression of endogenous GAP-43 mRNA and induction of GAP-43 promoter activity after LTP provided two insights. First, perforant path LTP likely involves the participation of mossy cells within the hilus. Of more than 10 different hilar cell types, the multipolar, spiny mossy cell is the most abundant (13). It is also the most likely candidate, because it alone satisfies four major requirements: (i) axons project into the inner molecular layer, (ii) projection to this layer is bilateral (17, 20), (iii) axons project up to 7 mm along the entire septotemporal axis (21), and (iv) cell bodies are located in the CHR and not the IHR. The GABAergic basket cell is another cell type that projects to the inner molecular layer bilaterally, but it is located in IHR (14, 20, 22), where no significant alteration of GAP-43 mRNA or transgene expression was observed. Therefore, it is unlikely that hilar basket cells are candidates for the elevated GAP-43 expression observed here. Indeed, hilar mossy cells in mouse identified by calretinin immunostaining (23, 24) are disposed in the hilus nearly identically to GAP-43-containing hilar cells.
We suggest that under in vivo conditions the dual inputs to granule cells from the perforant path and from axons of mossy cells act cooperatively to enhance LTP. The importance of the mossy cell input is suggested by a recent study from our laboratory on the effects of selective hilar lesions on perforant path LTP: kainate injected into the hilus, sparing granule cells but causing major hilar cell loss, caused perforant path LTP to decay to baseline 80–100 min after LTP induction, suggesting the necessity of hilar neurons in perforant path LTP (25).
A second insight provided by this study is that LTP likely activates the GAP-43 transcriptional mechanism. Recent studies have suggested that GAP-43 transcription is regulated in part by the basic helix–loop–helix (bHLH) protein family of transcription factors, which bind to E-box sequence elements on the GAP-43 promoter (26, 27). Indeed, it has been shown, using electrophoretic mobility-shift assays, that perforant path LTP produces a significant decrement in E-box binding. This binding is correlated with synaptic enhancement (26).
Over-expression of the neuron-specific GAP-43 leads to exuberant axonal growth (5, 28). This result gives support to the proposal that the potential for synaptic plasticity exists in those brain regions high in GAP-43 that are also associated with information storage processes (3). A preliminary study from our laboratory has recently shown that transgenic mice over-expressing GAP-43 demonstrate a nearly 2-fold increment in synaptic enhancement (29). Though this represents the initial observation that manipulation of GAP-43 can in fact alter LTP, it is entirely consistent with the findings of the present study, indicating that GAP-43 in fact regulates synaptic enhancement.
If constitutive levels of GAP-43 index capacity for synaptic change propelled by input-dependent soluble factors, what would be the consequence of input-dependent alteration in the constitutive levels themselves? In the case of granule cells, where levels are below detectability, the induction of GAP-43 by kainate has been shown to be positively correlated with new axonal growth (28). But in the hilar mossy cells that constitutively express GAP-43 at a high level, why is there a need for increased synthesis?
The initial enhancement of LTP, lasting hours, could be maintained in part by increased phosphorylation of existing GAP-43 in mossy cell axon terminals, possibly regulating increased neurotransmitter release in the inner molecular layer along with the presumed increased release in the perforant path (30). Thus, a cooperativity of the perforant path and mossy cell axon inputs may be important for enhanced responses in the first hours after tetanus. The proposed posttranslational modification by protein kinase C phosphorylation of GAP-43 may lead to subsequent proteolytic cleavage in the axonal terminal (31). In concert with kinase activation of IκB releasing synaptic NF-κB to activate transcription (32), this would yield an induction signal back to the cell nucleus (6).
The increase in GAP-43 synthesis may thus reflect the response to product decrement, suggesting a scenario for how mossy cells regulate their constitutive levels of GAP-43 in response to input-dependent activity. These considerations may also help explain, in part, the 48-hr time period between tetanus and elevated gene expression. That peak gene expression can have punctuated time points both hours and days after the initiating stimulus has also been observed consequent to songbird learning (33), mossy fiber sprouting (28), and limbic system activation (24, 34).
The results from the present study provide molecular evidence for a role of mossy cells in synaptic plasticity. Given the importance of GAP-43 as an intrinsic determinant of axonal growth (3), it is attractive to think that mossy cells with GAP-43 in the axon terminal may increase axonal arborization after LTP (Fig. 3B, stage V), thereby readjusting the associational microcircuitry of the dentate gyrus along the septotemporal axis. Such a revised circuit configuration may be part of the “binding” process (35) of memory formation.
Table 1.
Mean areal grain density of GAP-43 mRNA expression in the septal hilar region
| Region | Group | Areal grain density*
|
|||||
|---|---|---|---|---|---|---|---|
| Ipsilateral
|
Contralateral
|
||||||
| 1 day | 2 day | 3 day | 1 day | 2 day | 3 day | ||
| CHR | LTP† | 30.2 ± 2.0 | 38.8 ± 4.5 | 41.7 ± 4.3 | 29.9 ± 2.5 | 33.2 ± 3.2 | 31.7 ± 3.8 |
| LFC‡ | 29.3 ± 2.5 | 29.9 ± 0.9 | 28.5 ± 1.2 | 31.5 ± 1.7 | 30.5 ± 2.1 | 29.9 ± 2.3 | |
| IHR | LTP† | 29.9 ± 3.8 | 28.1 ± 3.7 | 29.3 ± 3.4 | 31.1 ± 3.6 | 31.6 ± 4.1 | 30.7 ± 4.9 |
| LFC‡ | 31.9 ± 1.4 | 29.3 ± 0.33 | 28.3 ± 4.3 | 28.2 ± 1.3 | 30.1 ± 1.1 | 31.0 ± 3.8 | |
Areal grain density (mean ± SEM) was quantified by image analysis (see Materials and Methods).
n = 5 for each time point.
n = 3 for each time point.
Acknowledgments
We are grateful to Dr. M. C. Fishman for providing transgenic mouse line 252 and P. Serrano and I. Cantallops for their helpful comments on this manuscript. This work was supported by the Northwestern University Graduate School (U.N.), the Japanese Ministry of Science (S.M.), and a National Institute of Mental Health MERIT award (A.R.).
ABBREVIATIONS
- LTP
long-term potentiation
- LFC
low-frequency control
- APV
dl-aminophosphonovalerate
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
- CHR
central hilar region
- IHR
infragranular hilar region
- PATE
promoter activity as indexed by transgene expression
Footnotes
In recent years we have used the term F1/GAP-43 to indicate both our initial observation (19) as well as its current common usage. However, in computer literature searching and in using transgenic mice with F1 generations, this designation has become less useful. Therefore, the protein is designated GAP-43, adopting the convention of our recent review (3).
References
- 1.Cole A J, Saffen D W, Baraban J M, Worley P F. Nature (London) 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- 2.Wisden W, Errington M L, Williams S, Dunnett S B, Waters C, Hitchcock D, Evan G, Bliss T V P, Hunt S P. Neuron. 1990;4:603–614. doi: 10.1016/0896-6273(90)90118-y. [DOI] [PubMed] [Google Scholar]
- 3.Benowitz L I, Routtenberg A. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 4.Lovinger D M, Akers R F, Nelson R B, Barnes C A, McNaughton B L, Routtenberg A. Brain Res. 1985;343:137–143. doi: 10.1016/0006-8993(85)91167-9. [DOI] [PubMed] [Google Scholar]
- 5.Aigner L, Arber S, Kapfhammer J P, Laux T, Schneider C, Botteri F, Brenner H-R, Caroni P. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 6.Meberg P J, Barnes C A, McNaughton B L, Routtenberg A. Proc Natl Acad Sci USA. 1993;90:12050–12054. doi: 10.1073/pnas.90.24.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanselow J, Grabczyk E, Ping J, Baetscher M, Teng S, Fishman M C. J Neurosci. 1994;14:499–510. doi: 10.1523/JNEUROSCI.14-02-00499.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNamara R K, Namgung U, Routtenberg A. Mol Brain Res. 1996;40:177–187. doi: 10.1016/0169-328x(96)00048-4. [DOI] [PubMed] [Google Scholar]
- 9.Namgung U, Valcourt E, Routtenberg A. Brain Res. 1995;689:85–92. doi: 10.1016/0006-8993(95)00531-t. [DOI] [PubMed] [Google Scholar]
- 10.Linden D J, Wong K L, Sheu F-S, Routtenberg A. Brain Res. 1988;458:142–146. doi: 10.1016/0006-8993(88)90506-9. [DOI] [PubMed] [Google Scholar]
- 11.Meberg P J, Routtenberg A. Neuroscience. 1991;45:721–733. doi: 10.1016/0306-4522(91)90284-u. [DOI] [PubMed] [Google Scholar]
- 12.Carmer S G, Swanson M R. J Am Stat Assoc. 1971;68:66–74. [Google Scholar]
- 13.Amaral D G. J Comp Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- 14.Freund T F, Buzsaki G. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Scharfman H E, Kunkel D D, Schwartzkroin P A. Neuroscience. 1990;37:693–707. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- 16.Cantallops I, Routtenberg A. Soc Neurosci Abstr. 1996;22:1932. [Google Scholar]
- 17.Zimmer J. J Comp Neurol. 1971;142:393–416. doi: 10.1002/cne.901420402. [DOI] [PubMed] [Google Scholar]
- 18.Swanson L W, Sawchenko P E, Cowan W M. J Neurosci. 1981;1:548–599. doi: 10.1523/JNEUROSCI.01-05-00548.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Routtenberg A. In: Neurobiology of Learning and Memory. Lynch G, McGaugh J, Weinberger N, editors; Lynch G, McGaugh J, Weinberger N, editors. New York: Guilford; 1984. pp. 479–490. [Google Scholar]
- 20.Ribak C E, Seress L, Amaral D G. J Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- 21.Buckmaster P S, Wenzel H J, Kunkel D D, Schwartzkroin P A. J Comp Neurol. 1996;366:270–292. doi: 10.1002/(sici)1096-9861(19960304)366:2<270::aid-cne7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Han Z-S, Buhl E H, Lorinczi Z, Somogyi P. Eur J Neurosci. 1993;5:395–410. doi: 10.1111/j.1460-9568.1993.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Fujise N, Kosaka T. Exp Brain Res. 1996;108:389–403. doi: 10.1007/BF00227262. [DOI] [PubMed] [Google Scholar]
- 24.Blasco-Ibanez J M, Freund T F. Hippocampus. 1997;7:307–320. doi: 10.1002/(SICI)1098-1063(1997)7:3<307::AID-HIPO6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 25.Serrano, P. A. & Routtenberg, A. (1997) Soc. Neurosci. Abstr., in press.
- 26.Kinney W R, McNamara R K, Valcourt E, Routtenberg A. Mol Brain Res. 1996;38:25–36. doi: 10.1016/0169-328x(95)00287-3. [DOI] [PubMed] [Google Scholar]
- 27.Chiaramello A, Neuman T, Peavy D R, Zuber M X. J Biol Chem. 1996;271:22035–22043. doi: 10.1074/jbc.271.36.22035. [DOI] [PubMed] [Google Scholar]
- 28.Cantallops I, Routtenberg A. J Comp Neurol. 1996;366:303–319. doi: 10.1002/(SICI)1096-9861(19960304)366:2<303::AID-CNE9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Routtenberg, A. & Serrano, P. (1997) Soc. Neurosci. Abstr. 23, in press.
- 30.Dekker L V, DeGraan P N, Versteeg D H, Oestreicher A B, Gispen W H. J Neurochem. 1989;52:24–30. doi: 10.1111/j.1471-4159.1989.tb10893.x. [DOI] [PubMed] [Google Scholar]
- 31.Zwiers H, Gispen W H, Kleine L, Mahler H R. Neurochem Res. 1982;7:127–137. doi: 10.1007/BF00965051. [DOI] [PubMed] [Google Scholar]
- 32.Meberg P J, Kinney W R, Valcourt E G, Routtenberg A. Mol Brain Res. 1996;38:179–190. doi: 10.1016/0169-328x(95)00229-l. [DOI] [PubMed] [Google Scholar]
- 33.Chew S J, Vicario D S, Nottebohm F. Science. 1996;274:1909–1914. doi: 10.1126/science.274.5294.1909. [DOI] [PubMed] [Google Scholar]
- 34.Guzowski J F, McGaugh J L. Proc Natl Acad Sci USA. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckmaster P S, Schwartzkroin P A. Hippocampus. 1994;4:393–402. doi: 10.1002/hipo.450040402. [DOI] [PubMed] [Google Scholar]
- 36.Scharfman H E. J Neurophysiol. 1995;74:179–194. doi: 10.1152/jn.1995.74.1.179. [DOI] [PubMed] [Google Scholar]