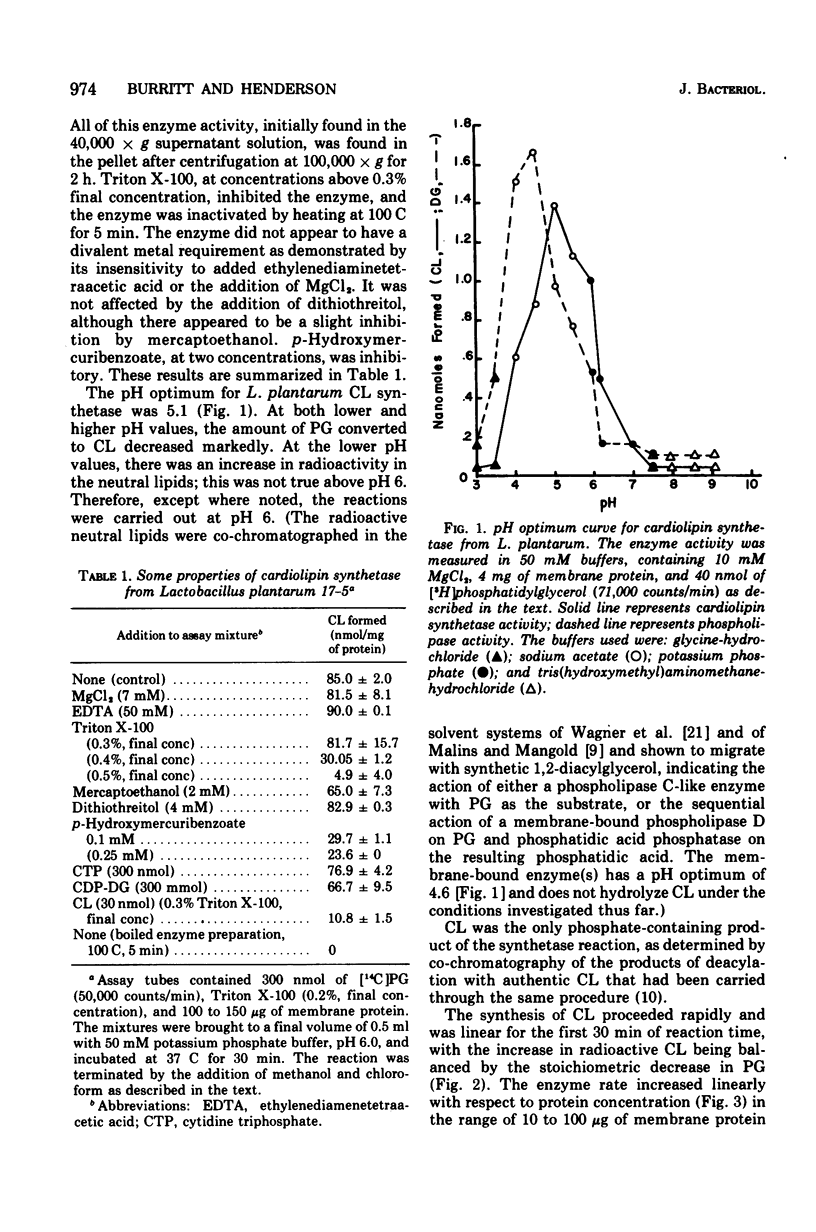
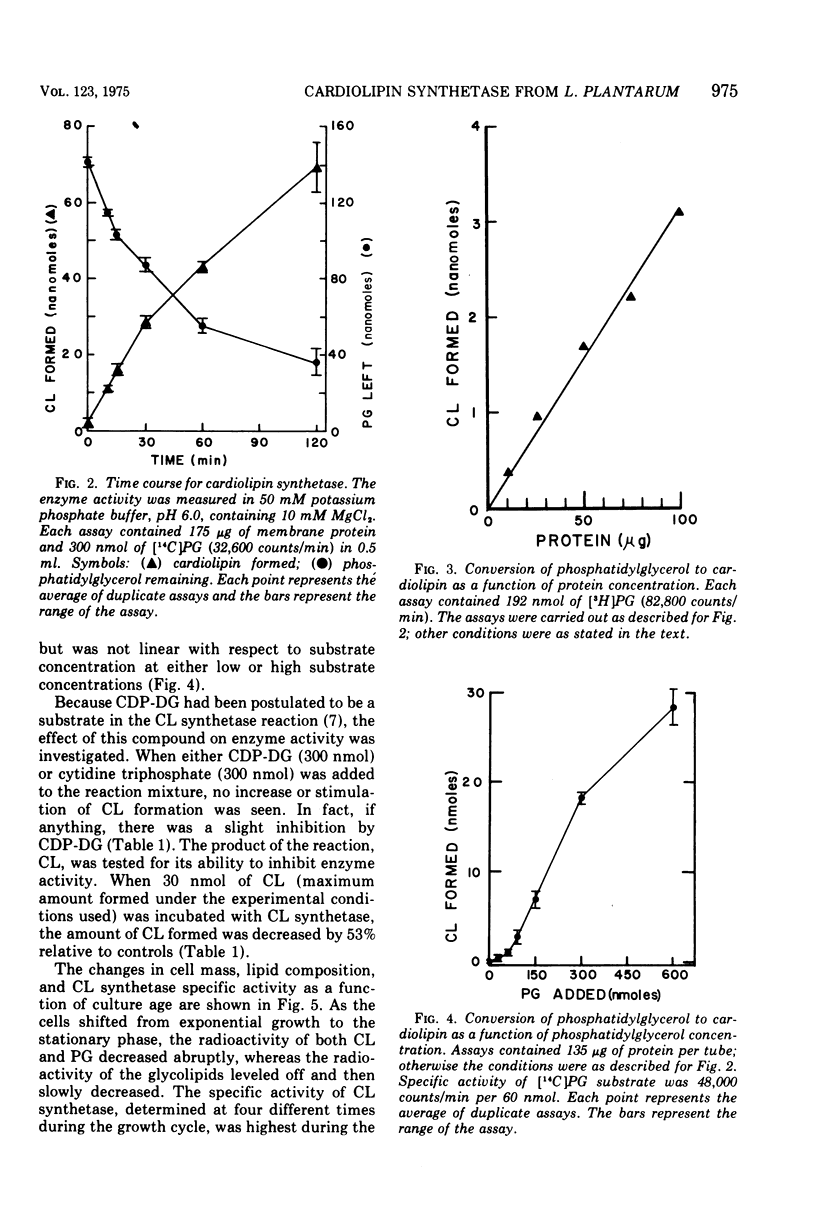
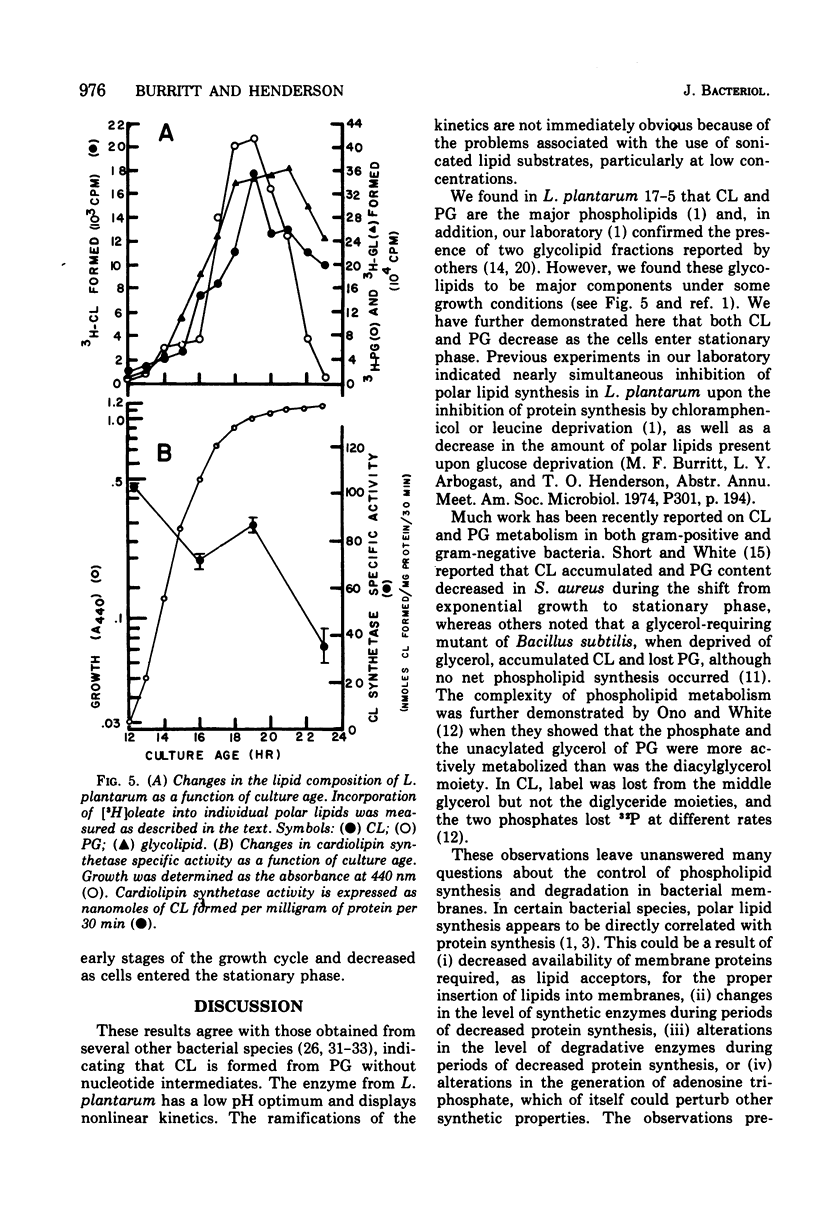
Abstract
Cardiolipin (CL) synthetase of Lactobacillus plantarum 17-5 catalyzed the stoichiometric conversion of 2 mol of phosphatidylglycerol to 1 mol of CL. The enzyme activity was linear with time for 30 min at 37 C and with protein concentration between 20 and 200 mug of protein per ml. The enzyme was membrane associated, had a pH optimum of 5.1 in phosphate buffer, and was not stimulated by Mg2+, and the activity was not affected by the addition of ethylenediaminetetraacetic acid, cytidine diphosphate diglyceride, or cytidine triphosphate. The reaction was inhibited about 95% by Triton X-100 (0.5% final concentration) and by CL, the end product of the reaction. The activity of this enzyme was studied as a function of growth. The CL synthetase specific activity was highest during the early and midexponential growth phases, as was the cellular content of CL. The results demonstrate a correlation between enzyme-specific activity and lipid content of the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbogast L. Y., Henderson T. O. Effect of inhibition of protein synthesis on lipid metabolism in Lactobacillus plantarum. J Bacteriol. 1975 Sep;123(3):962–971. doi: 10.1128/jb.123.3.962-971.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Siervo A. J., Salton M. R. Biosynthesis of cardiolipin in the membranes of Micrococcus lysodeikticus. Biochim Biophys Acta. 1971 Jul 13;239(2):280–292. doi: 10.1016/0005-2760(71)90174-3. [DOI] [PubMed] [Google Scholar]
- Glaser M., Bayer W. H., Bell R. M., Vagelos P. R. Regulation of macromolecular biosynthesis in a mutant of Escherichia coli defective in membrane phospholipid biosynthesis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):385–389. doi: 10.1073/pnas.70.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson T. O., McNeill J. J., Tove S. B. Folic acid involvement in cyclopropane fatty acid synthesis in lactobacilli. J Bacteriol. 1965 Nov;90(5):1283–1287. doi: 10.1128/jb.90.5.1283-1287.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg C. B., Kennedy E. P. Mechanism of the enzymatic synthesis of cardiolipin in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Mar;69(3):648–651. doi: 10.1073/pnas.69.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIYASU J. Y., PIERINGER R. A., PAULUS H., KENNEDY E. P. The biosynthesis of phosphatidylglycerol. J Biol Chem. 1963 Jul;238:2293–2298. [PubMed] [Google Scholar]
- Kirkpatrick D. S., Bishop S. H. Simplified wet ash procedure for total phosphorus analysis of organophosphonates in biological samples. Anal Chem. 1971 Oct;43(12):1707–1709. doi: 10.1021/ac60306a046. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARUO B., BENSON A. A. Cyclic glycerophosphate formation from the glycerolphosphatides. J Biol Chem. 1959 Feb;234(2):254–256. [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Ono Y., White D. C. Consequences of the inhibition of cardiolipin metabolism in Haemophilus parainfluenzae. J Bacteriol. 1971 Dec;108(3):1065–1071. doi: 10.1128/jb.108.3.1065-1071.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. G., Patton S. Two-dimensional thin-layer chromatography of polar lipids from milk and mammary tissue. J Lipid Res. 1967 Nov;8(6):696–698. [PubMed] [Google Scholar]
- Short S. A., White D. C. Biosynthesis of cardiolipin from phosphatidylglycerol in Staphylococcus aureus. J Bacteriol. 1972 Feb;109(2):820–826. doi: 10.1128/jb.109.2.820-826.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Kodicek E. The structure of bactoprenol, a lipid formed by lactobacilli from mevalonic acid. Biochem J. 1966 Apr;99(1):123–127. doi: 10.1042/bj0990123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunaitis E., Cronan J. E., Jr Characterization of the cardiolipin synthetase activity of Escherichia coli envelopes. Arch Biochem Biophys. 1973 Apr;155(2):420–427. doi: 10.1016/0003-9861(73)90132-x. [DOI] [PubMed] [Google Scholar]
- VORBECK M. L., MARINETTI G. V. SEPARATION OF GLYCOSYL DIGLYCERIDES FROM PHOSPHATIDES USING SILICIC ACID COLUMN CHROMATOGRAPHY. J Lipid Res. 1965 Jan;6:3–6. [PubMed] [Google Scholar]
- Weeks G., Wakil S. J. Studies on the control of fatty acid metabolism. II. The inhibition of fatty acid synthesis in Lactobacillus plantarum by exogenous fatty acid. J Biol Chem. 1970 Apr 25;245(8):1913–1921. [PubMed] [Google Scholar]


