Abstract
The source of metabolic energy for the accumulation of methionine in cells of Escherichia coli was shown to differ from that for proline uptake. In contrast to proline uptake, methionine accumulation was sensitive to arsenate, and relatively resistant to azide or dinitrophenol. Adenosine triphosphatase mutant strains also differentiated between the two systems, consistent with the conclusion that, although proline uptake is driven directly by the energized membrane state, methionine uptake is not. Methionine transport is similar to that of other osmotic shock-sensitive systems in its direct utilization of adenosine 5'-triphosphate or a related compound as energy source.
Full text
PDF

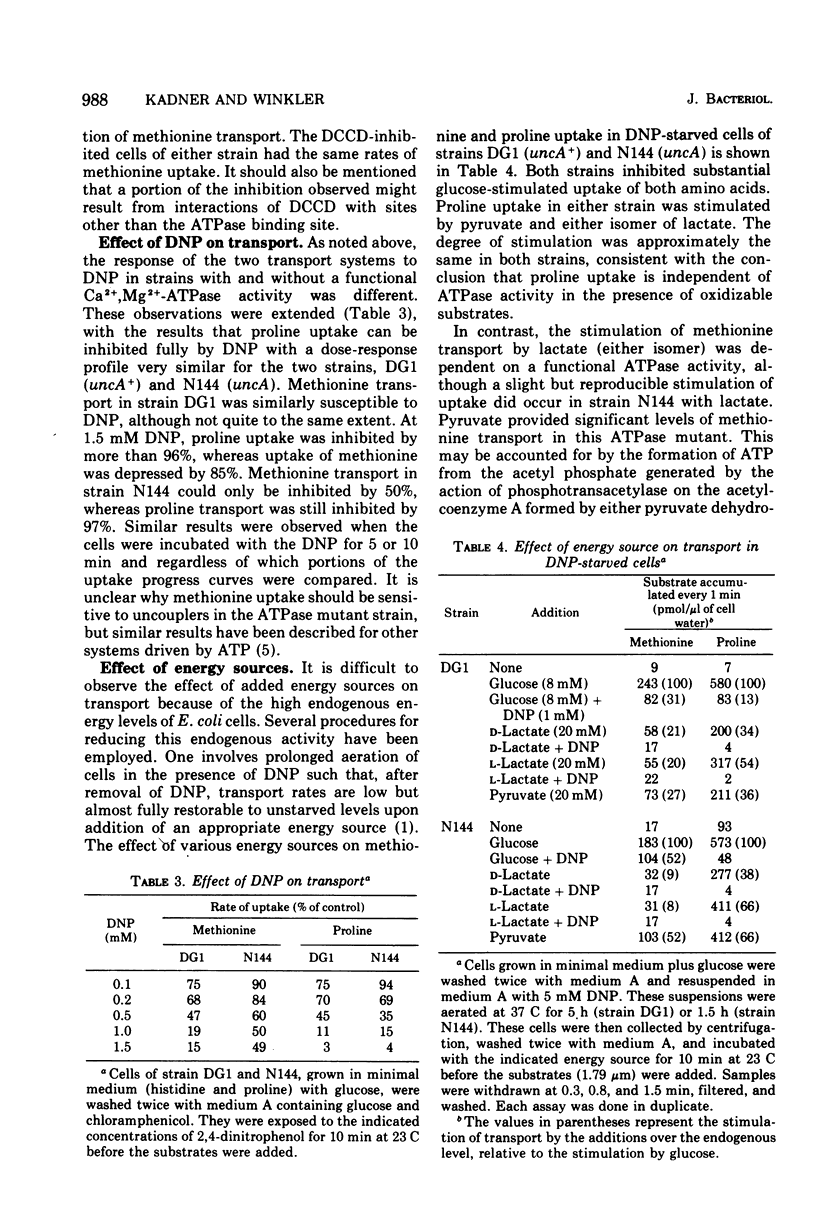



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell J. L. Energetics of glycylglycine transport in Escherichia coli. J Bacteriol. 1974 Oct;120(1):139–146. doi: 10.1128/jb.120.1.139-146.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J. Mechanism of energy coupling for transport of D-ribose in Escherichia coli. J Bacteriol. 1974 Oct;120(1):295–303. doi: 10.1128/jb.120.1.295-303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor K. A., Schairer H. U., Renz D., Overath P. Active transport of beta-galactosides by a mutant of Escherichia coli defective in heme synthesis. Eur J Biochem. 1974 Jun 15;45(2):451–456. doi: 10.1111/j.1432-1033.1974.tb03569.x. [DOI] [PubMed] [Google Scholar]
- Gutnick D. L., Kanner B. I., Postma P. W. Oxidative phosphorylation in mutants of Escherichia coli defective in energy transduction. Biochim Biophys Acta. 1972 Nov 17;283(2):217–222. doi: 10.1016/0005-2728(72)90237-x. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- Kadner R. J. Transport systems for L-methionine in Escherichia coli. J Bacteriol. 1974 Jan;117(1):232–241. doi: 10.1128/jb.117.1.232-241.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
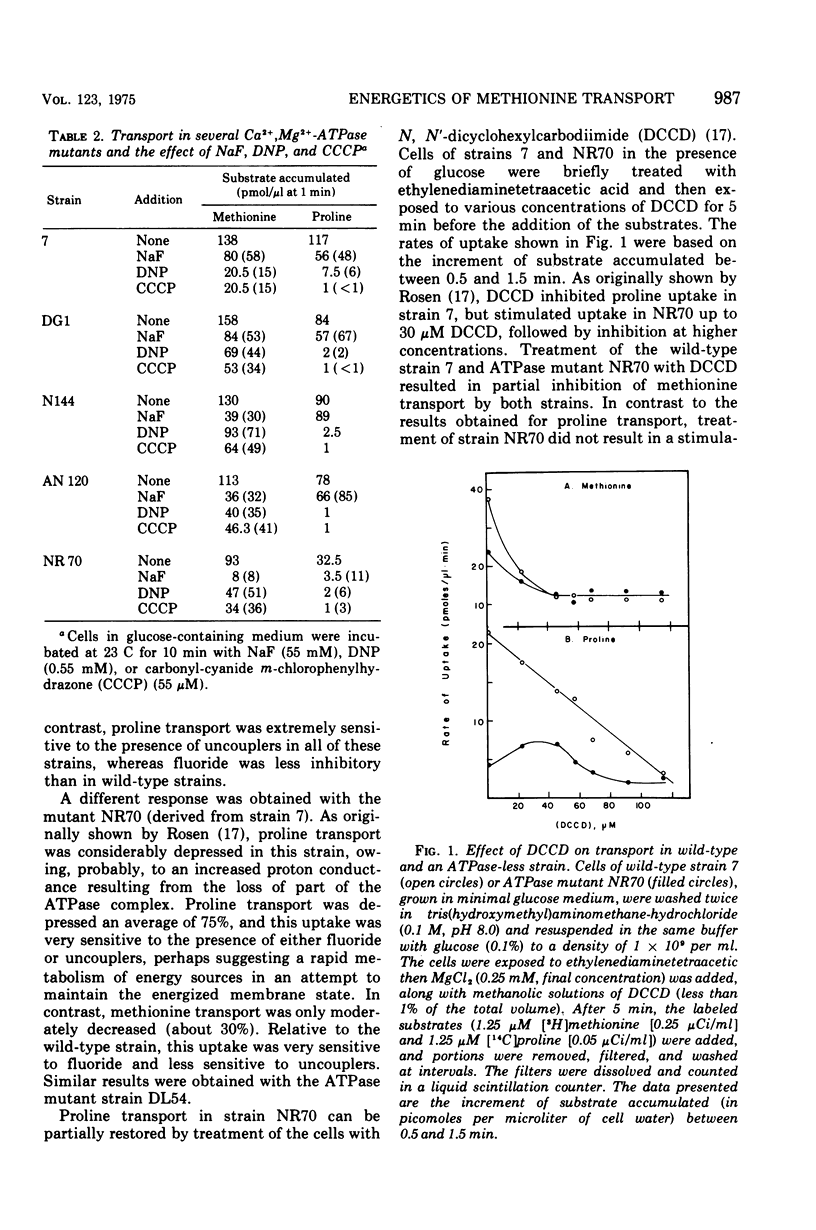
- Kadner R. J., Watson W. J. Methionine transport in Escherichia coli: physiological and genetic evidence for two uptake systems. J Bacteriol. 1974 Aug;119(2):401–409. doi: 10.1128/jb.119.2.401-409.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Lombardi F. J., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. 8. The transport of amino acids by membranes prepared from Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):7844–7857. [PubMed] [Google Scholar]
- Maloney P. C., Kashket E. R., Wilson T. H. A protonmotive force drives ATP synthesis in bacteria. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3896–3900. doi: 10.1073/pnas.71.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezioso G., Hong J. S., Kerwar G. K., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. XII. Active transport by a mutant of Escherichia coli uncoupled for oxidative phosphorylation. Arch Biochem Biophys. 1973 Feb;154(2):575–582. doi: 10.1016/0003-9861(73)90011-8. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Restoration of active transport in an Mg2+-adenosine triphosphatase-deficient mutant of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1124–1129. doi: 10.1128/jb.116.3.1124-1129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Shallenberger M. K. Coupling of energy to active transport of amino acids in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2663–2667. doi: 10.1073/pnas.69.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. Source of energy for the Escherichia coli galactose transport systems induced by galactose. J Bacteriol. 1974 Nov;120(2):866–871. doi: 10.1128/jb.120.2.866-871.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


