Abstract
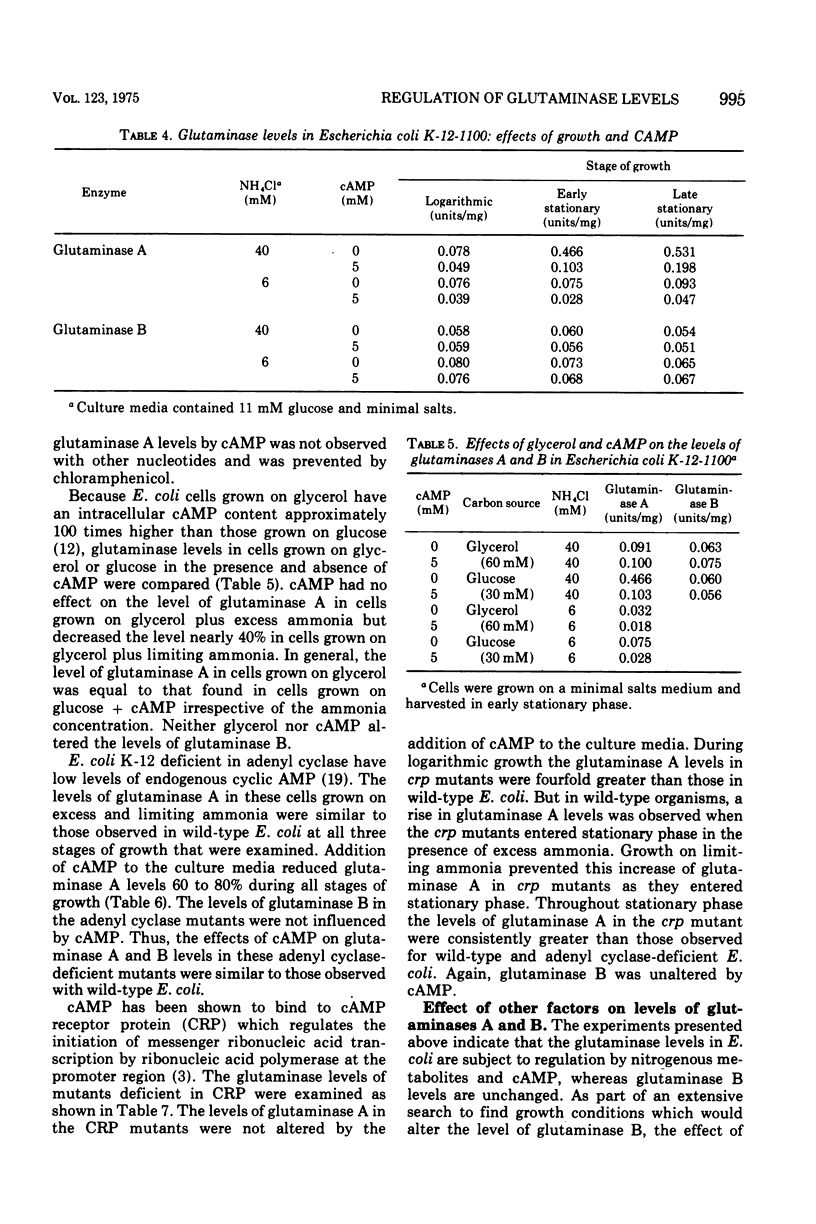
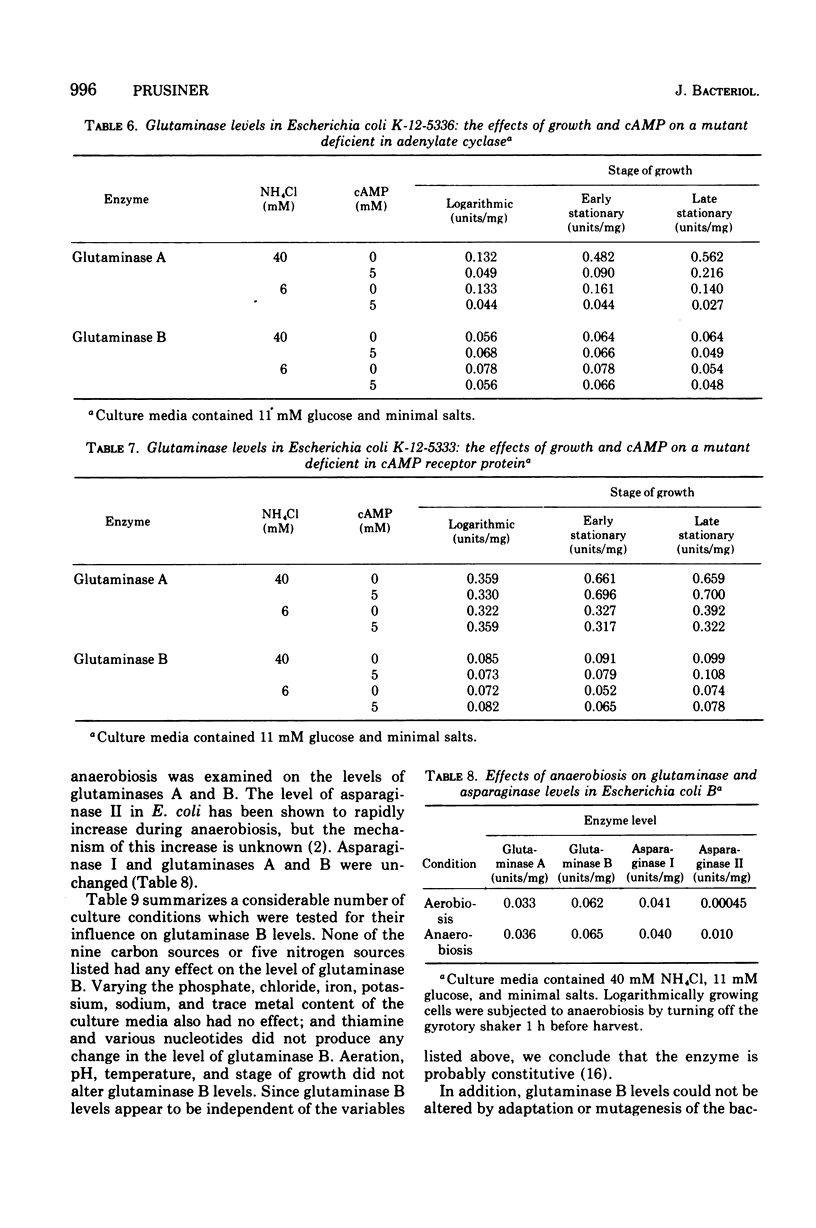
Nitrogenous metabolites, cyclic adenosine 3':5'-monophosphate (cAMP), and the stage of culture growth all influence the levels of glutaminase A in Escherichia coli, but no variables in culture conditions alter the levels of glutaminase B. Growth of E. coli on culture media containing glucose and excess ammonia results in a rise in the level of glutaminase A as the cultures enter stationary phase; this rise is abolished by ammonia limitation. cAMP or glycerol reduce the level of glutaminase A. In mutants deficient in cAMP receptor protein, glutaminase A levels are unchanged by cAMP, but they are still susceptible to regulation by ammonia. We consider glutaminase B to be a constitutive enzyme, since its levels appear independent of nutritional conditions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cedar H., Schwartz J. H. Production of L-asparaginase II by Escherichia coli. J Bacteriol. 1968 Dec;96(6):2043–2048. doi: 10.1128/jb.96.6.2043-2048.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Anderson W., Nissley P., Gottesman M., Pastan I., Perlman R. Lac DNA, RNA polymerase and cyclic AMP receptor protein, cyclic AMP, lac repressor and inducer are the essential elements for controlled lac transcription. Nat New Biol. 1971 Jun 2;231(22):139–142. doi: 10.1038/newbio231139a0. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G., Banerjee S. A mutation increasing the amount of a constitutive enzyme in Escherichia coli, glucose 6-phosphate dehydrogenase. J Mol Biol. 1971 Feb 28;56(1):183–194. doi: 10.1016/0022-2836(71)90093-3. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. A role of aminoacyl-tRNA in the regulation of protein breakdown in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Feb;68(2):362–366. doi: 10.1073/pnas.68.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S. Induction and repression of glutamic acid decarboxylase in Escherichia coli. Biochim Biophys Acta. 1962 Dec 31;61:953–962. doi: 10.1016/0926-6550(62)90011-7. [DOI] [PubMed] [Google Scholar]
- Hartman S. C. Glutaminase of Escherichia coli. I. Purification and general catalytic properties. J Biol Chem. 1968 Mar 10;243(5):853–863. [PubMed] [Google Scholar]
- Kozlov E. A., Kovalenko N. A., Mardashev S. R. Ochistka i nekotory svoistva glutaminazy Clostridium welchii SR-12. Biokhimiia. 1972 Jan-Feb;37(1):56–64. [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- MEISTER A., LEVINTOW L., GREENFIELD R. E., ABENDSCHEIN P. A. Hydrolysis and transfer reactions catalyzed by omega-amidase preparations. J Biol Chem. 1955 Jul;215(1):441–460. [PubMed] [Google Scholar]
- Mardashev S. R., Eremenko V. V., Nikolaev A. Ia. Identifikatsiia Pseudomonas sp. i vliianie uslovii vyrashchivaniia na asparaginaznuiu i glutaminaznuiu aktivnosti. Mikrobiologiia. 1970 Jan-Feb;39(1):11–17. [PubMed] [Google Scholar]
- Mardashev S. R., Nikolaev A. Ia, Evseev L. P., Eremenko V. V. Induktsiia asparaginaznoi i gliutaminaznoi aktivnostei u Pseudomonas sp. asparaginovoi i gliutaminovoi kislotami. Biokhimiia. 1967 Sep-Oct;32(5):1093–1098. [PubMed] [Google Scholar]
- Pardee A. B., Benz E. J., Jr, St Peter D. A., Krieger J. N., Meuth M., Trieshmann H. W., Jr Hyperproduction and purification of nicotinamide deamidase, a microconstitutive enzyme of Escherichia coli. J Biol Chem. 1971 Nov 25;246(22):6792–6796. [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):151–157. doi: 10.1016/0006-291x(69)90893-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S., Miller R. E., Valentine R. C. Adenosine 3':5'-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2922–2926. doi: 10.1073/pnas.69.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S., Milner L. A rapid radioactive assay for glutamine synthetase, glutaminase, asparagine synthetase, and asparaginase. Anal Biochem. 1970 Oct;37(2):429–438. doi: 10.1016/0003-2697(70)90069-2. [DOI] [PubMed] [Google Scholar]
- Prusiner S., Stadtman E. R. On the regulation of glutaminase in E. coli: metabolite control. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1474–1481. doi: 10.1016/0006-291x(71)90186-0. [DOI] [PubMed] [Google Scholar]
- Roberts J., Holcenberg J. S., Dolowy W. C. Isolation, crystallization, and properties of Achromobacteraceae glutaminase-asparaginase with antitumor activity. J Biol Chem. 1972 Jan 10;247(1):84–90. [PubMed] [Google Scholar]
- Schutt H., Holzer H. Biological function of the ammonia-induced inactivation of glutamine synthetase in Escherichia coli. Eur J Biochem. 1972 Mar 15;26(1):68–72. doi: 10.1111/j.1432-1033.1972.tb01740.x. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Reeves J. Y., Broome J. D. Two L-asparaginases from E. coli and their action against tumors. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1516–1519. doi: 10.1073/pnas.56.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R., Shapiro B. M., Kingdon H. S., Woolfolk C. A., Hubbard J. S. Cellular regulation of glutamine synthetase activity in Escherichia coli. Adv Enzyme Regul. 1968;6:257–289. doi: 10.1016/0065-2571(68)90017-4. [DOI] [PubMed] [Google Scholar]
- Varricchio F. Control of glutaminase synthesis in Escherichia coli. Arch Mikrobiol. 1972;81(3):234–238. doi: 10.1007/BF00412241. [DOI] [PubMed] [Google Scholar]
- Willetts N. S. Intracellular protein breakdown in growing cells of Escherichia coli. Biochem J. 1967 May;103(2):462–466. doi: 10.1042/bj1030462. [DOI] [PMC free article] [PubMed] [Google Scholar]


