Abstract
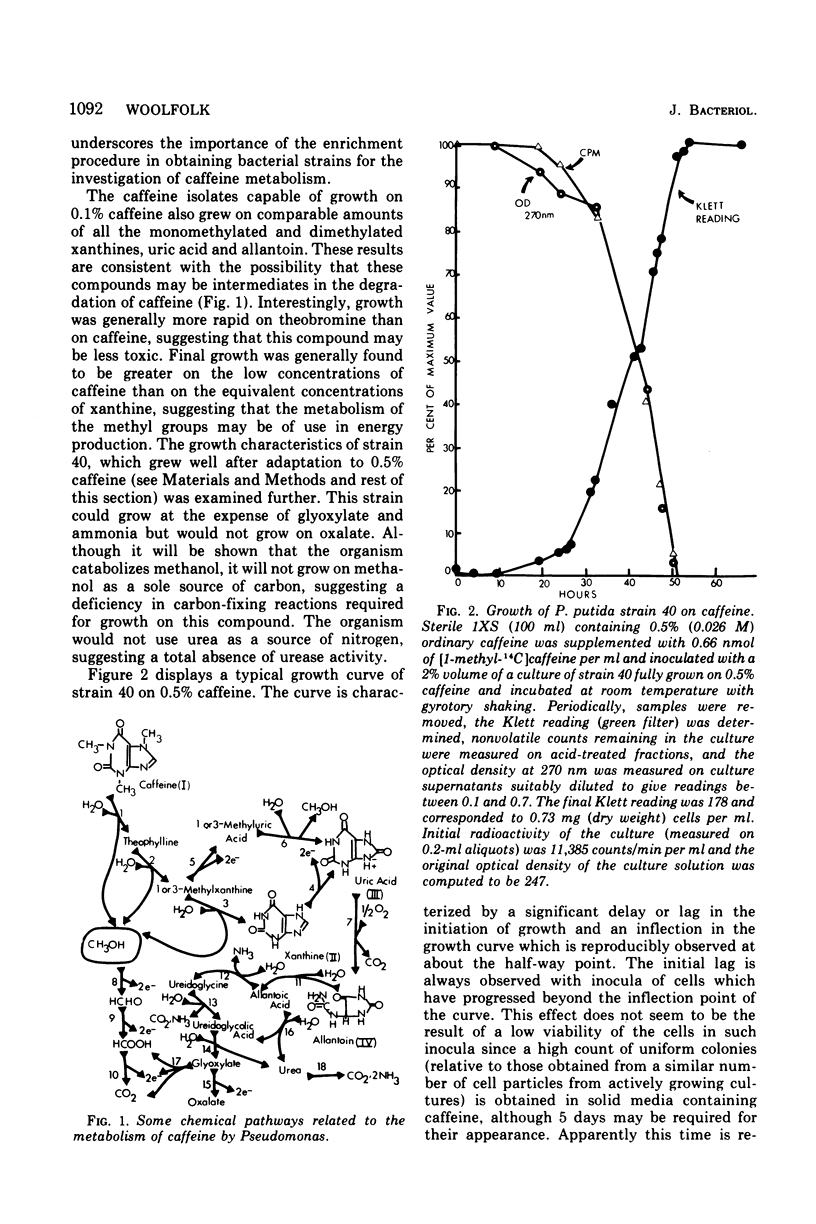
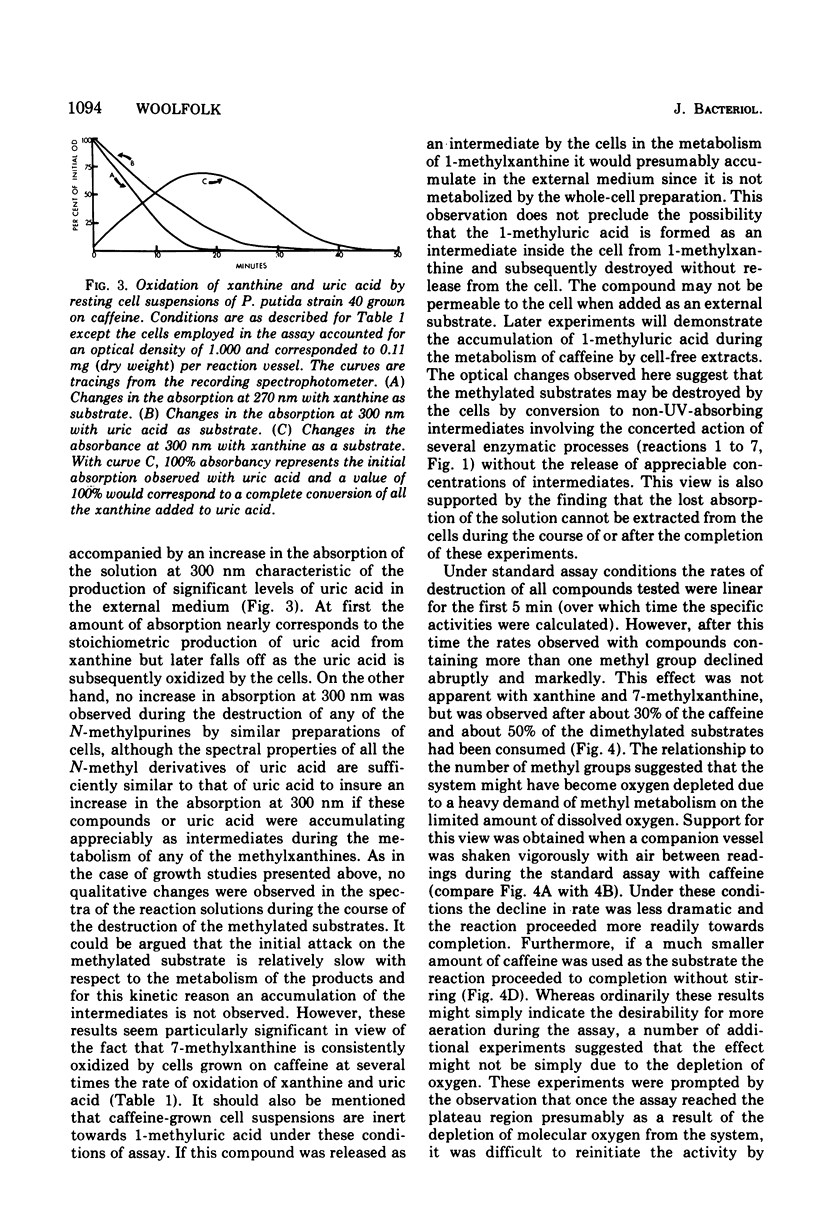
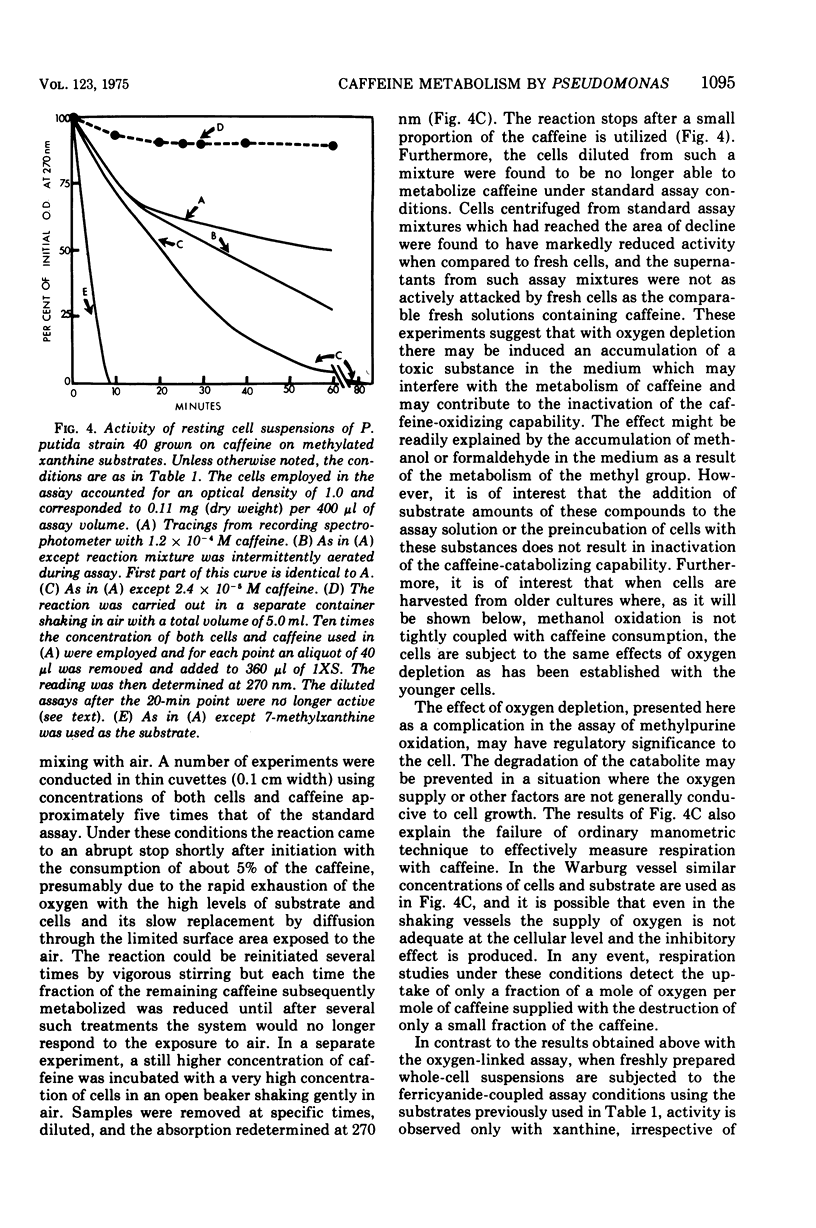
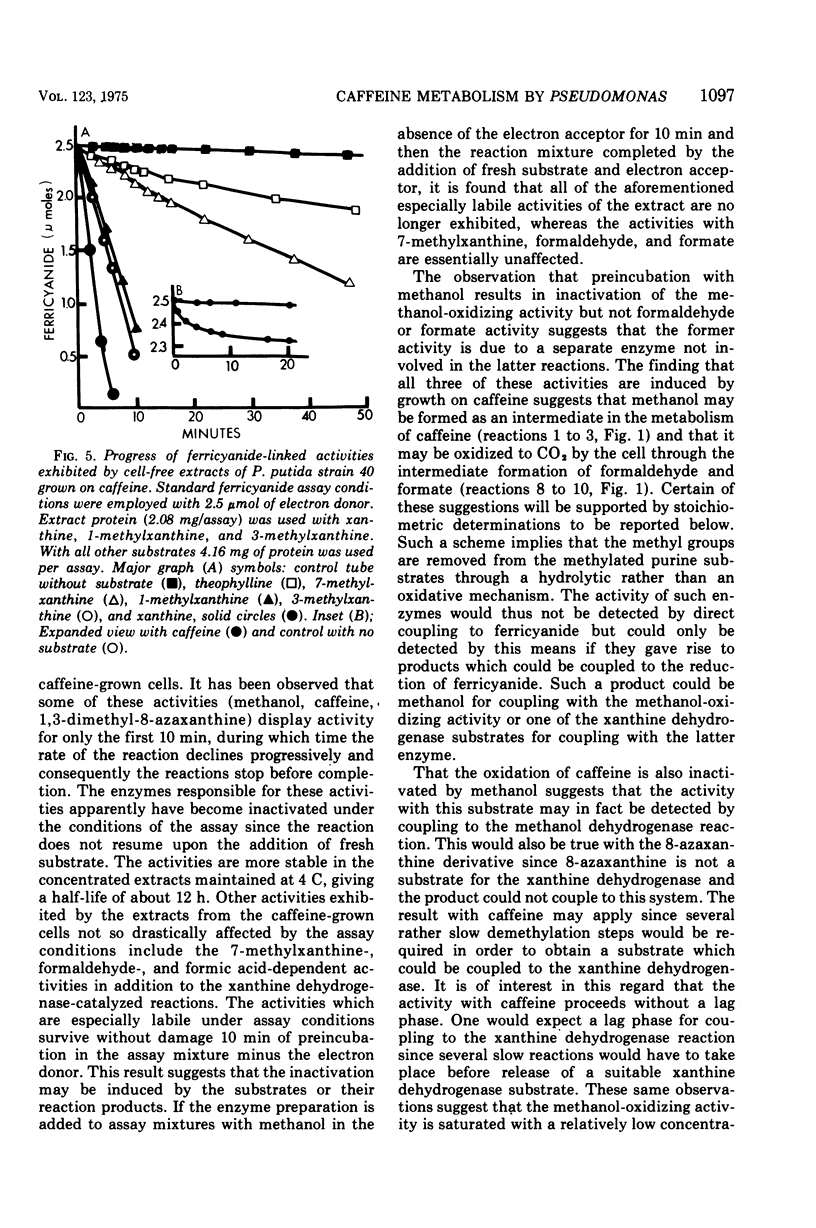
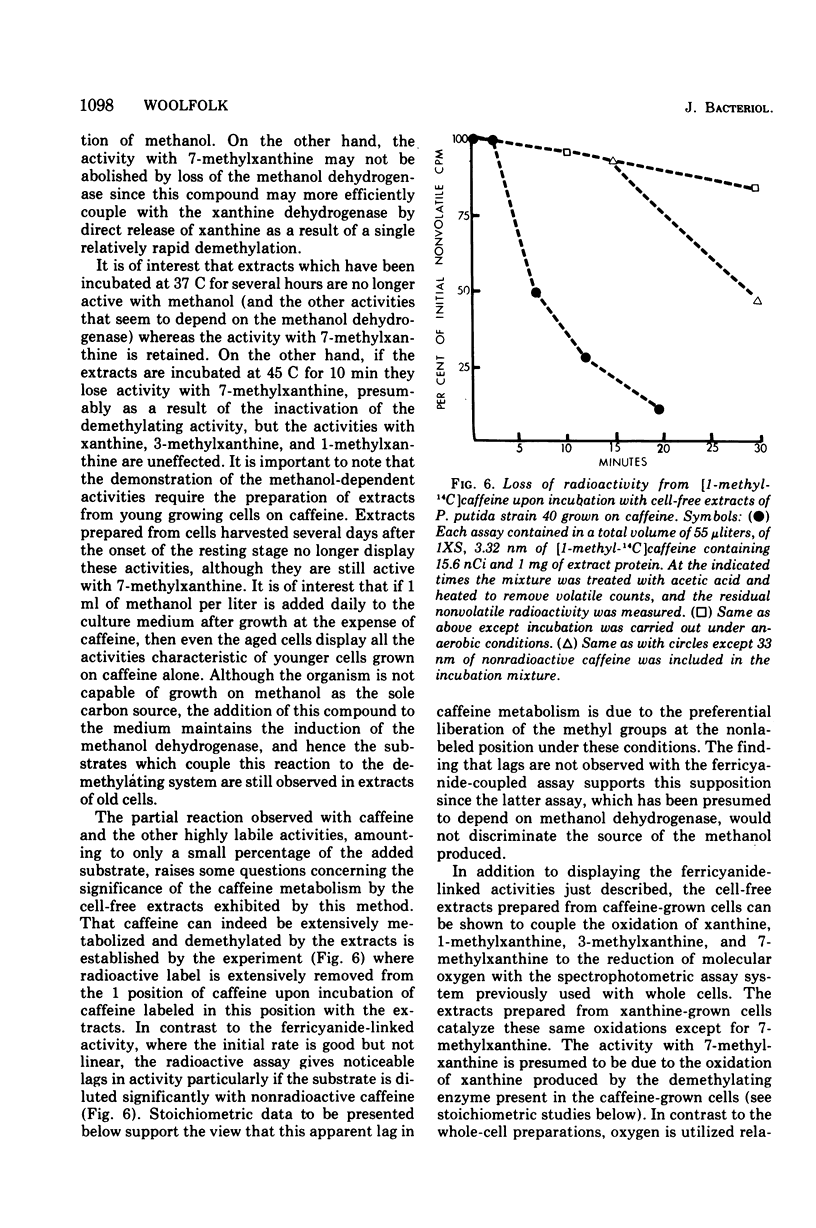
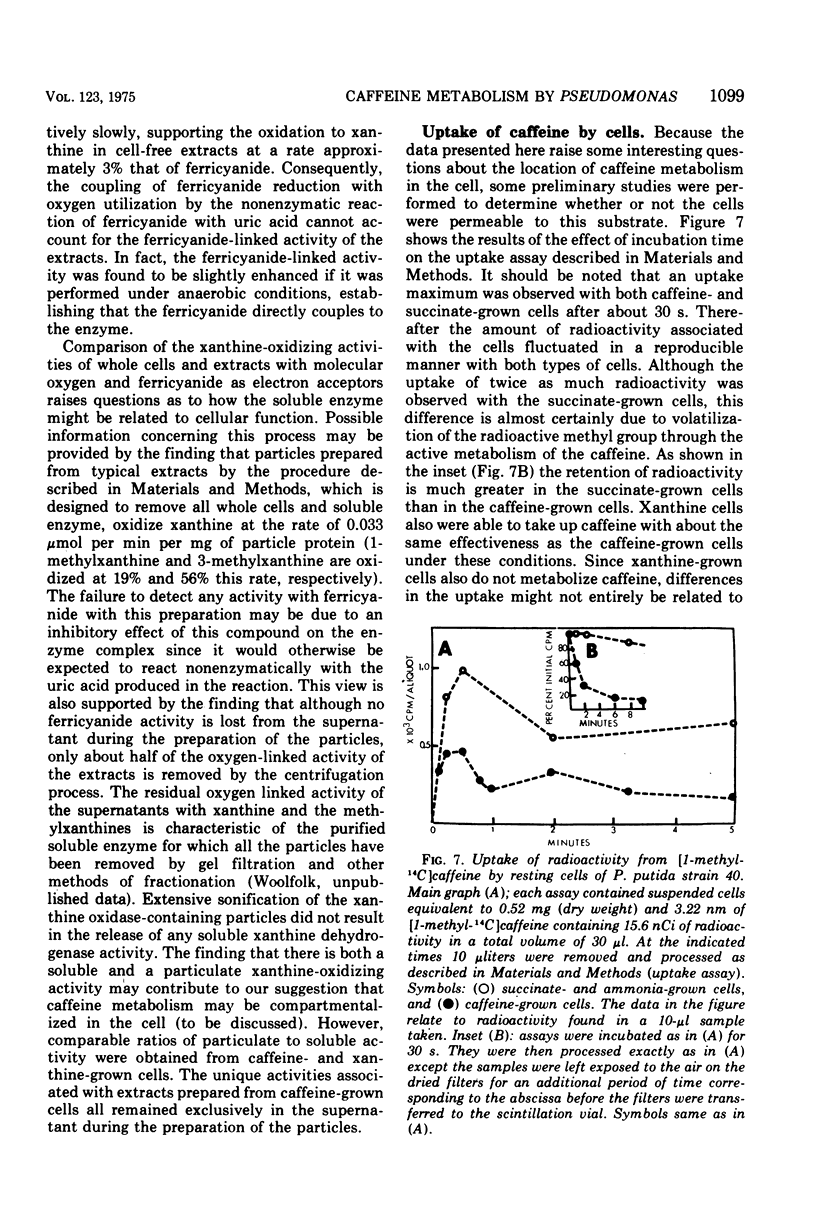
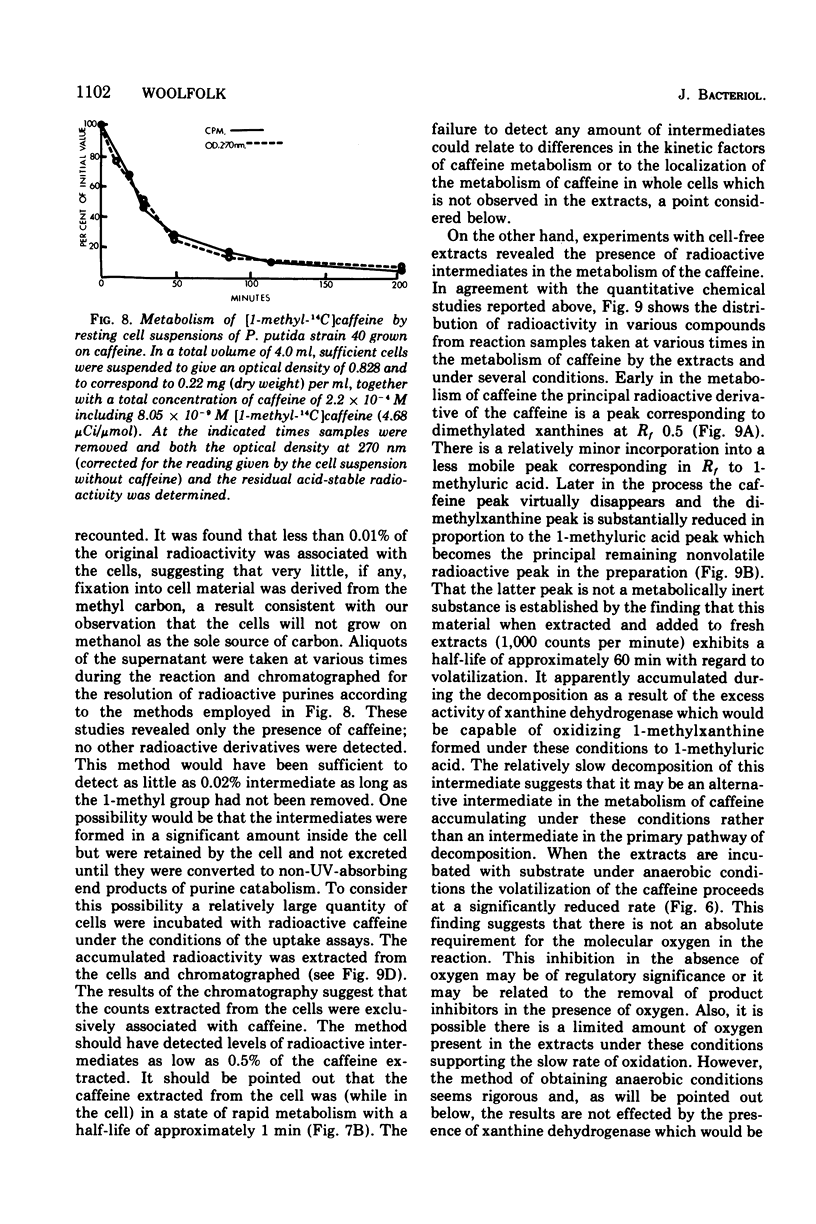
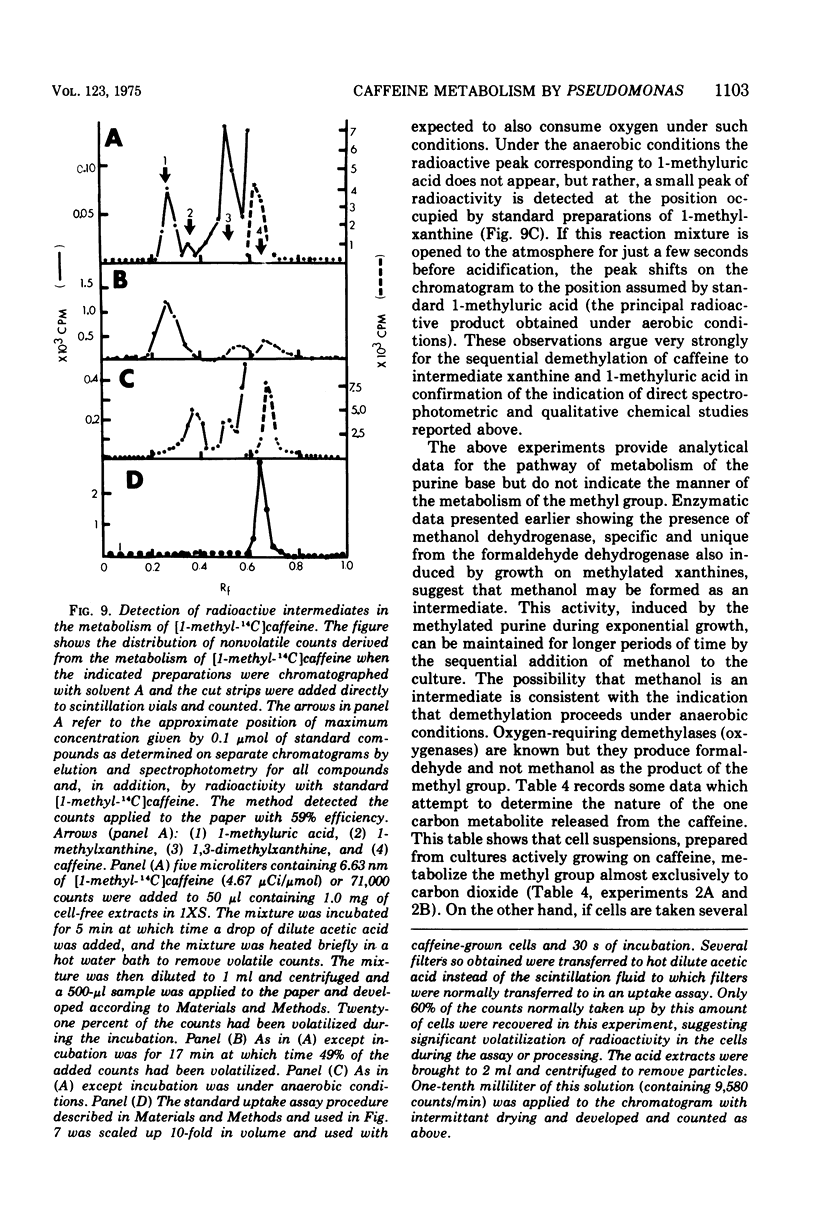
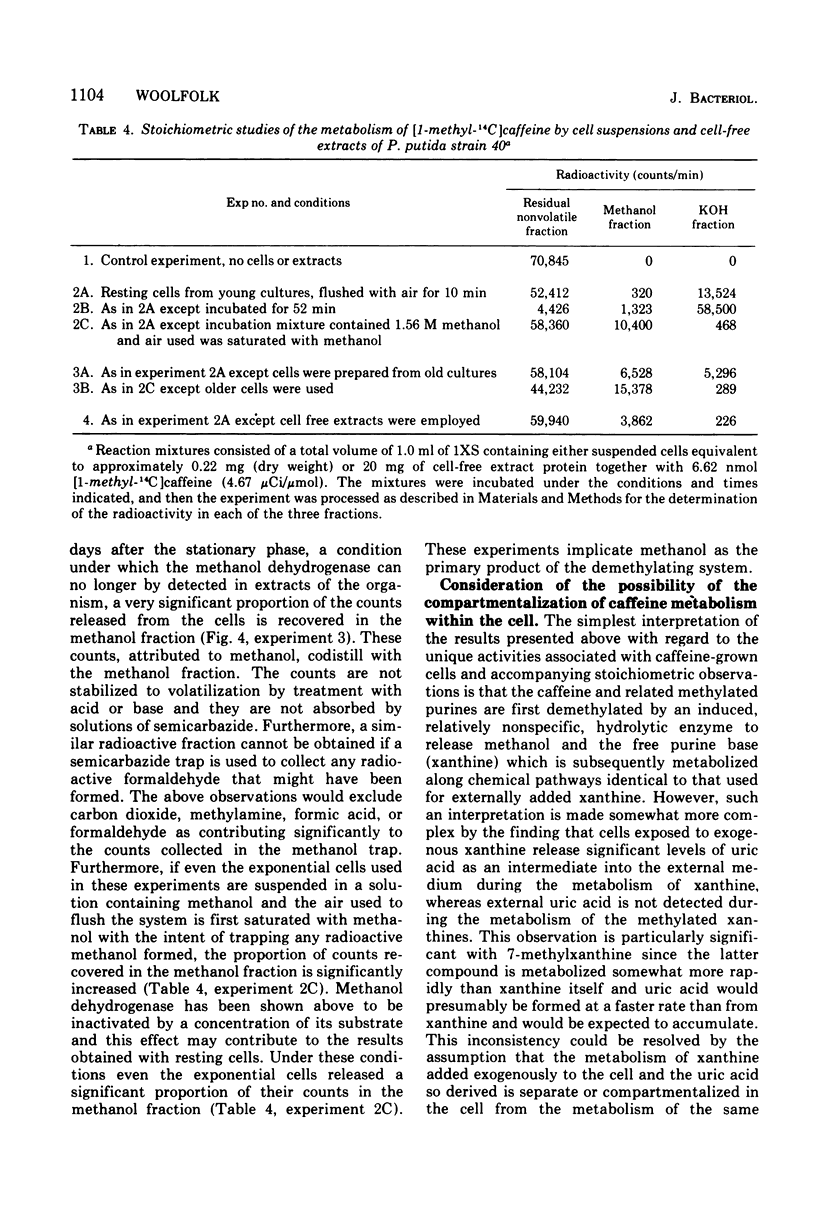
Pseudomonas putida, strain 40, originally isolated by enrichment on caffeine as the sole source of carbon and nitrogen, has been developed to grow on 0.5% caffeine. The organism will grow on any N-methyl derivative of xanthine containing one or more methyl groups at the 1, 3, or 7 positions. An investigation of the activities of resting cell suspensions and cell-free preparations together with the detection of metabolic intermediates suggest that caffeine is first metabolized by the action of an enzyme which is capable of hydrolytically removing all three methyl groups with the production of methanol and free xanthine. The methanol presumably is oxidized to the final product, CO2, through the sequential action of methanol, formaldehyde, and formate dehydrogenases, which are induced by growth on caffeine. Furthermore, the xanthine would seem to be channeled through conventional pathways of purine degradation through the action of xanthine dehydrogenase and uricase, both induced by growth on caffeine. However, a variety of data suggests that the metabolism of caffeine may be compartmentalized in the cell and metabolized separately from externally added xanthine. Additional studies indicated that the cell is permeable to the methylxanthines. The significance of these findings is discussed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Burger M. Cyclic 3';5' adenosine monophosphate-phosphodiesterase and the release of catabolite repression of beta-galactosidase by exogenous cyclic 3';5' adenosine monophosphate in Escherichia coli. Biochem Biophys Res Commun. 1971 Apr 2;43(1):174–182. doi: 10.1016/s0006-291x(71)80103-1. [DOI] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 2. The methanol-oxidizing enzyme of Pseudomonas sp. M 27. Biochem J. 1964 Sep;92(3):614–621. doi: 10.1042/bj0920614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGMANN F., DIKSTEIN S., HENIS Y. Studies on uric acid and related compounds. IV. The specificity of bacterial xanthine oxidases. J Biol Chem. 1957 Jan;224(1):67–77. [PubMed] [Google Scholar]
- BERGMANN F., DIKSTEIN S. Studies on uric acid and related compounds. III. Observations on the specificity of mammalian xanthine oxidases. J Biol Chem. 1956 Dec;223(2):765–780. [PubMed] [Google Scholar]
- BERGMANN F., UNGAR-WARON H., KWIETNY-GOVRIN H., GOLDBERG H., LEON S. Some specific reactions of the purine-oxidizing system of Pseudomonas aeruginosa. Biochim Biophys Acta. 1962 Apr 2;55:512–522. doi: 10.1016/0006-3002(62)90984-8. [DOI] [PubMed] [Google Scholar]
- Bergmann F., Mahler A., Burger-Rachamimov H., Diller D. Mechanism of uptake of purines by Pseudomonas aeruginosa. Biochemistry. 1969 Feb;8(2):457–465. doi: 10.1021/bi00830a001. [DOI] [PubMed] [Google Scholar]
- Bergmann F., Ungar-Waron H., Kwietny-Govrin H. Action of 8-azaguanine and 8-azaxanthine on Pseudomonas aeruginosa. Biochem J. 1964 May;91(2):270–276. doi: 10.1042/bj0910270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. J. On the regulation of glycogen metabolism in Tetrahymena. Arch Biochem Biophys. 1970 Mar;137(1):65–74. doi: 10.1016/0003-9861(70)90411-x. [DOI] [PubMed] [Google Scholar]
- Burg A. W., Stein M. E. Urinary excretion of caffeine and its metabolites in the mouse. Biochem Pharmacol. 1972 Apr 1;21(7):909–922. doi: 10.1016/0006-2952(72)90396-6. [DOI] [PubMed] [Google Scholar]
- CAMPBELL L. L., Jr The mechanism of allantoin degradation by a Pseudomonas. J Bacteriol. 1954 Nov;68(5):598–603. doi: 10.1128/jb.68.5.598-603.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIUSA W., BARBIROLI G. CRYSTALLINE LENS AND BLOOD AS SUBSTRATES OF GENERAL APPLICATION FOR THE STUDY OF TRANSMETHYLATION PROCESSES. Exp Eye Res. 1963 Jul;2:265–267. doi: 10.1016/s0014-4835(63)80045-7. [DOI] [PubMed] [Google Scholar]
- CORNISH H. H., CHRISTMAN A. A. A study of the metabolism of theobromine, theophylline, and caffeine in man. J Biol Chem. 1957 Sep;228(1):315–323. [PubMed] [Google Scholar]
- DAVOREN P. R., SUTHERLAND E. W. THE EFFECT OF L-EPINEPHRINE AND OTHER AGENTS ON THE SYNTHESIS AND RELEASE OF ADENOSINE 3',5'-PHOSPHATE BY WHOLE PIGEON ERYTHROCYTES. J Biol Chem. 1963 Sep;238:3009–3015. [PubMed] [Google Scholar]
- DIKSTEIN S., BERGMANN F., CHAIMOVITZ M. Studies on uric acid and related compounds. II. Paper chromatography of substituted xanthines and uric acids. J Biol Chem. 1956 Jul;221(1):239–251. [PubMed] [Google Scholar]
- FRANKE W., HAHN G. E. Untersuchungen zum bakteriellen Purin-Abbau. II. Uber den Abbau von Amino-, Oxy - und Methyl-purinen durch Pseudomonas aeruginosa (B. pyocyaneum). Hoppe Seylers Z Physiol Chem. 1955 Jun 30;301(1-2):90–106. [PubMed] [Google Scholar]
- FRANKE W., HAHN G. E. Untersuchungen zum bakteriellen Purinabbau. I. Uber den Harnsäureabbau durch Pseudomonas aeurginosa (Badt. pyocyaneum). Hoppe Seylers Z Physiol Chem. 1955;299(1):15–38. [PubMed] [Google Scholar]
- FRIDOVICH I., HANDLER P. Xanthine oxidase. IV. Participation of iron in internal electron transport. J Biol Chem. 1958 Dec;233(6):1581–1585. [PubMed] [Google Scholar]
- Grigg G. W. Effects of coumarin, pyronin Y, 6,9-dimethyl 2-methylthiopurine and caffeine on excision repair and recombination repair in Escherichia coli. J Gen Microbiol. 1972 Apr;70(2):221–230. doi: 10.1099/00221287-70-2-221. [DOI] [PubMed] [Google Scholar]
- HENDERSON J. F., MAZEL P. DEMETHYLATION OF PURINE ANALOGS BY MICROSOMAL ENZYMES FROM MOUSE LIVER. Biochem Pharmacol. 1964 Feb;13:207–210. doi: 10.1016/0006-2952(64)90138-8. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH A. L. The metabolism of methylpurines by Escherichia coli. I. Tracer studies. J Biol Chem. 1956 Mar;219(1):181–188. [PubMed] [Google Scholar]
- Khanna K. L., Rao G. S., Cornish H. H. Metabolism of caffeine- 3 H in the rat. Toxicol Appl Pharmacol. 1972 Dec;23(4):720–730. doi: 10.1016/0041-008x(72)90112-3. [DOI] [PubMed] [Google Scholar]
- Kurtzman R. H., Jr, Schwimmer S. Caffeine removal from growth media by microorganisms. Experientia. 1971 Apr 15;27(4):481–482. doi: 10.1007/BF02137327. [DOI] [PubMed] [Google Scholar]
- Lombrozo L., Mitoma C. Effect of caffeine on hepatic microsomal cytochrome P-450. Biochem Pharmacol. 1970 Jul;19(7):2317–2323. doi: 10.1016/0006-2952(70)90130-9. [DOI] [PubMed] [Google Scholar]
- Martin Y. C., Hansch C. Influence of hydrophobic character on the relative rate of oxidation of drugs by rat liver microsomes. J Med Chem. 1971 Sep;14(9):777–779. doi: 10.1021/jm00291a600. [DOI] [PubMed] [Google Scholar]
- Nair K. G. Purification and properties of 3',5'-cyclic nucleotide phosphodiesterase from dog heart. Biochemistry. 1966 Jan;5(1):150–157. doi: 10.1021/bi00865a020. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- Nielsen L. D., Monard D., Rickenberg H. V. Cyclic 3',5'-adenosine monophosphate phosphodiesterase of Escherichia coli. J Bacteriol. 1973 Nov;116(2):857–866. doi: 10.1128/jb.116.2.857-866.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabayashi T., Ide M. Effect of dipicolinic acid on bacterial cyclic 3,5'-nucleotide phosphodiesterase. Biochim Biophys Acta. 1970 Oct 14;220(1):124–126. doi: 10.1016/0005-2744(70)90236-6. [DOI] [PubMed] [Google Scholar]
- PALMER G., BRAY R. C., BEINERT H. DIRECT STUDIES ON THE ELECTRON TRANSFER SEQUENCE IN XANTHINE OXIDASE BY ELECTRON PARAMAGNETIC RESONANCE SPECTROSCOPY. I. TECHNIQUES AND DESCRIPTION OF SPECTRA. J Biol Chem. 1964 Aug;239:2657–2666. [PubMed] [Google Scholar]
- Putrament A., Baranowska H., Biliński T., Prazmo W. On the specificity of caffeine effects. Inhibition by caffeine of RNA and protein synthesis in yeast and Escherichia coli. Mol Gen Genet. 1972;118(4):373–379. doi: 10.1007/BF00333572. [DOI] [PubMed] [Google Scholar]
- RAJ C. V., DHALA S. EFFECT OF NATURALLY OCCURRING XANTHINES ON BACTERIA. I. ANTIMICROBIAL ACTION AND POTENTIATING EFFECT ON ANTIBIOTIC SPECTRA. Appl Microbiol. 1965 May;13:432–436. doi: 10.1128/am.13.3.432-436.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer S., Kurtzman R. H., Jr, Heftmann E. Caffeine metabolism by Penicillium roqueforti. Arch Biochem Biophys. 1971 Nov;147(1):109–113. doi: 10.1016/0003-9861(71)90315-8. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Trijbels F., Vogels G. D. Allantoate and ureidoglycolate degradation by Pseudomonas aeruginosa. Biochim Biophys Acta. 1967 Jan 11;132(1):115–126. doi: 10.1016/0005-2744(67)90197-0. [DOI] [PubMed] [Google Scholar]
- Vízdalová M., Janovská E., Zhestjanikov V. D. Effect of dark-repair inhibitors on the survival of Escherichia coli B under different post-irradiation conditions. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;20(1):49–59. doi: 10.1080/09553007114550861. [DOI] [PubMed] [Google Scholar]
- Warren R. N. Metabolism of xanthine alkaloids in man. J Chromatogr. 1969 Apr 8;40(3):468–469. doi: 10.1016/s0021-9673(01)96689-0. [DOI] [PubMed] [Google Scholar]
- Woolfolk C. A., Woolfolk B. S., Whiteley H. R. 2-oxypurine dehydrogenase from Micrococcus aerogenes. I. Isolation, specificity, and some chemical and physical properties. J Biol Chem. 1970 Jun;245(12):3167–3178. [PubMed] [Google Scholar]



