Abstract
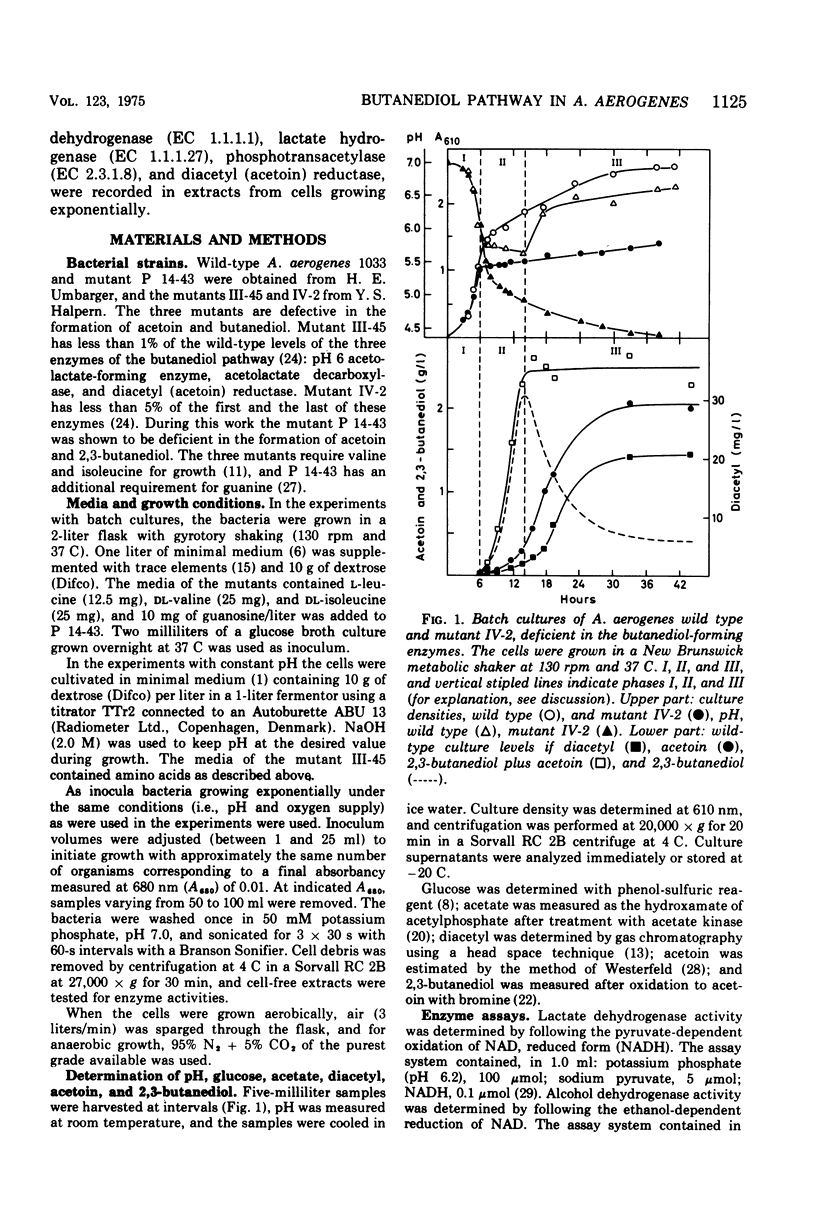
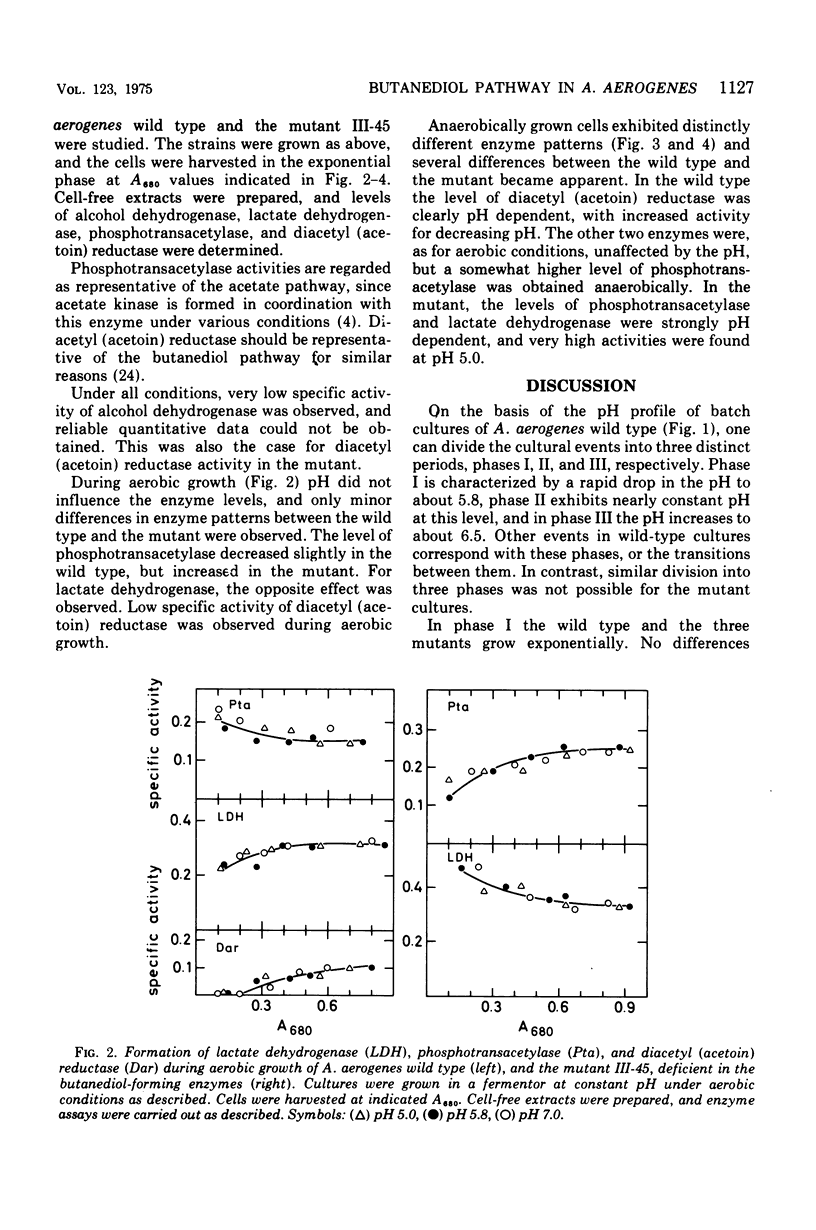
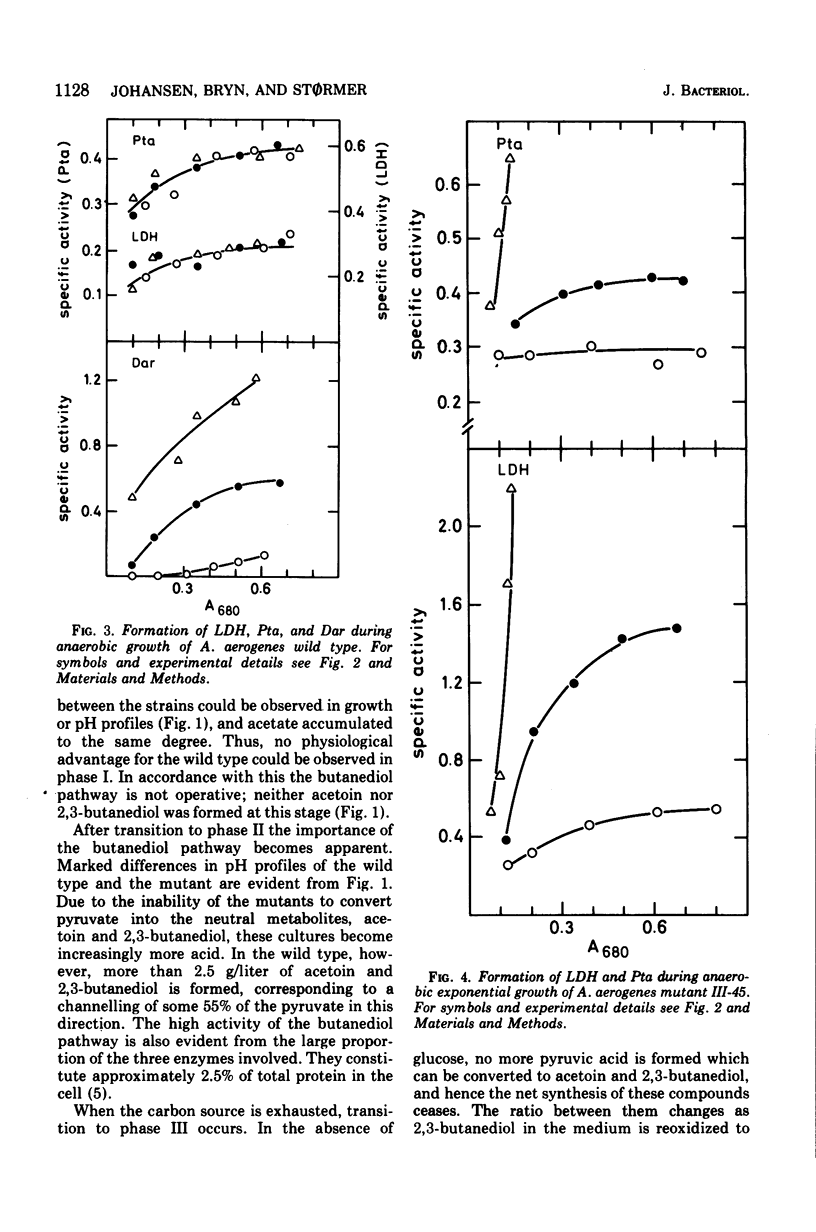
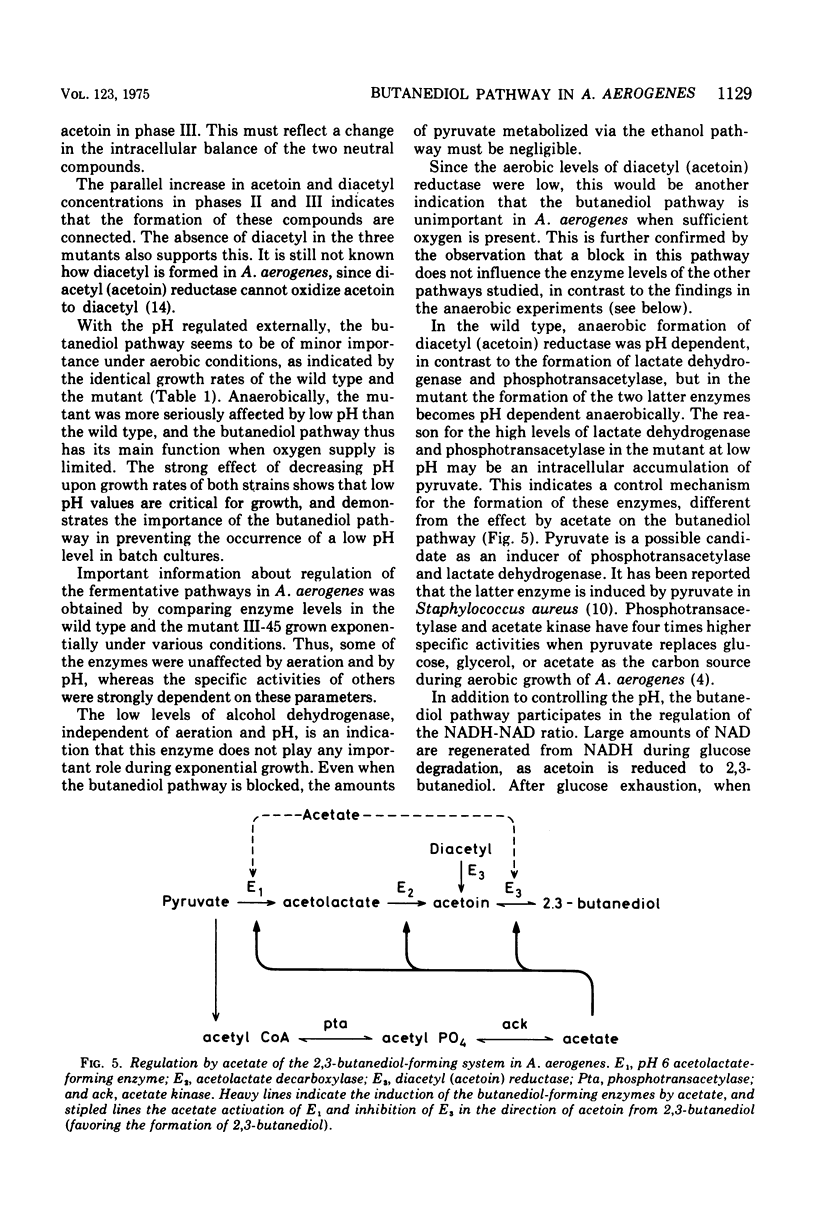
Aerobacter (Enterobacter) aerogenes wild type and three mutants deficient in the formation of acetoin and 2,3-butanediol were grown in a glucose minimal medium. Culture densities, pH, and diacetyl, acetoin, and 2,3-butanediol levels were recorded. The pH in wild-type cultures dropped from 7.0 to 5.8, remained constant while acetoin and 2,3-butanediol were formed, and increased to pH 6.5 after exhaustion of the carbon source. More 2,3-butanediol than acetoin was formed initially, but after glucose exhaustion reoxidation to acetoin occurred. The three mutants differed from the wild type in yielding acid cultures (pH below 4.5). The wild type and one of the mutants were grown exponentially under aerobic and anaerobic conditions with the pH fixed at 7.0, 5.8, and 5.0, respectively. Growth rates decreased with decreasing pH values. Aerobically, this effect was weak, and the two strains were affected to the same degree. Under anaerobic conditions, the growth rates were markedly inhibited at a low pH, and the mutant was slightly more affected than the wild type. Levels of alcohol dehydrogenase were low under all conditions, indicating that the enzyme plays no role during exponential growth. The levels of diacetyl (acetoin) reductase, lactate dehydrogenase, and phosphotransacetylase were independent of the pH during aerobic growth of the two strains. Under anaerobic conditions, the formation of diacetyl (acetoin) reductase was pH dependent, with much higher levels of the enzyme at pH 5.0 than at pH 7.0. Lactate dehydrogenase and phosphotransacetylase revealed the same pattern of pH-dependent formation in the mutant, but not in the wild type.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth J. M., Kornberg H. L. The anaplerotic fixation of carbon dioxide by Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):179–188. doi: 10.1098/rspb.1966.0063. [DOI] [PubMed] [Google Scholar]
- BERGMEYER H. U., HOLZ G., KLOTZSCH H., LANG G. PHOSPHOTRANSACETYLASE AUS CLOSTRIDIUM KLUYVERI. ZUECHTUNG DES BACTERIUMS, ISOLIERUNG, KRISTALLISATION UND EIGENSCHAFTEN DES ENZYMS. Biochem Z. 1963;338:114–121. [PubMed] [Google Scholar]
- Branen A. L., Keenan T. W. Diacetyl reductase of Lactobacillus casei. Can J Microbiol. 1970 Oct;16(10):947–951. doi: 10.1139/m70-162. [DOI] [PubMed] [Google Scholar]
- Brown T. D., Pereira C. R., Stormer F. C. Studies of the acetate kinase-phosphotransacetylase and the butanediol-forming systems in Aerobacter aerogenes. J Bacteriol. 1972 Dec;112(3):1106–1111. doi: 10.1128/jb.112.3.1106-1111.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryn K., Hetland O., Stormer F. C. The reduction of diacetyl and acetoin in Aerobacter aerogenes. Evidence for one enzyme catalyzing both reactions. Eur J Biochem. 1971 Jan 1;18(1):116–119. doi: 10.1111/j.1432-1033.1971.tb01221.x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez V., Burgos J., Martín R. Pigeon liver diacetyl reductase: purification and some properties. Biochim Biophys Acta. 1974 Jun 18;350(2):253–262. doi: 10.1016/0005-2744(74)90499-9. [DOI] [PubMed] [Google Scholar]
- Gabriel M. A., Jabara H., al-Khalidi U. A. Metabolism of acetoin in mammalian liver slices and extracts. Interconversion with butane-2,3-diol and biacetyl. Biochem J. 1971 Oct;124(4):793–800. doi: 10.1042/bj1240793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrard W., Lascelles J. Regulation of Staphylococcus aureus lactate dehydrogenase. J Bacteriol. 1968 Jan;95(1):152–156. doi: 10.1128/jb.95.1.152-156.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Even-Shoshan A. Further evidence for two distinct acetolactate synthetases in Aerobacter aerogenes. Biochim Biophys Acta. 1967 Jul 11;139(2):502–504. doi: 10.1016/0005-2744(67)90053-8. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Pirt S. J. The influence of dissolved oxygen concentration on the respiration and glucose metabolism of Klebsiella aerogenes during growth. J Gen Microbiol. 1967 Feb;46(2):193–211. doi: 10.1099/00221287-46-2-193. [DOI] [PubMed] [Google Scholar]
- Johansen L., Larsen S. H., Stormer F. C. Diacetyl (acetoin) reductase from Aerobacter aerogenes. Kinetic studies of the reduction of diacetyl to acetoin. Eur J Biochem. 1973 Apr 2;34(1):97–99. doi: 10.1111/j.1432-1033.1973.tb02733.x. [DOI] [PubMed] [Google Scholar]
- KOGUT M., PODOSKI E. P. Oxidative pathways in a fluorescent Pseudomonas. Biochem J. 1953 Dec;55(5):800–811. doi: 10.1042/bj0550800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen S. H., Stormer F. C. Diacetyl (acetoin) reductase from Aerobacter aerogenes. Kinetic mechanism and regulation by acetate of the reversible reduction of acetoin to 2,3-butanediol. Eur J Biochem. 1973 Apr 2;34(1):100–106. doi: 10.1111/j.1432-1033.1973.tb02734.x. [DOI] [PubMed] [Google Scholar]
- Loken J. P., Stormer F. C. Acetolactate decarboxylase from Aerobacter aerogenes. Purification and properties. Eur J Biochem. 1970 May 1;14(1):133–137. doi: 10.1111/j.1432-1033.1970.tb00270.x. [DOI] [PubMed] [Google Scholar]
- McPhedran P., Sommer B., Lin E. C. CONTROL OF ETHANOL DEHYDROGENASE LEVELS IN AEROBACTER AEROGENES. J Bacteriol. 1961 Jun;81(6):852–857. doi: 10.1128/jb.81.6.852-857.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- Silber P., Chung H., Gargiulo P., Schulz H. Purification and properties of a diacetyl reductase from Escherichia coli. J Bacteriol. 1974 Jun;118(3):919–927. doi: 10.1128/jb.118.3.919-927.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speckman R. A., Collins E. B. Separation of diacetyl, acetoin, and 2,3-butylene glycol by salting-out chromatography. Anal Biochem. 1968 Jan;22(1):154–160. doi: 10.1016/0003-2697(68)90269-8. [DOI] [PubMed] [Google Scholar]
- Stormer F. C. 2,3-Butanediol biosynthetic system in Aerobacter aerogenes. Methods Enzymol. 1975;41:518–532. doi: 10.1016/s0076-6879(75)41108-9. [DOI] [PubMed] [Google Scholar]
- Stormer F. C. Evidence for induction of the 2,3-butanediol-forming enzymes in Aerobacter aerogenes. FEBS Lett. 1968 Nov;2(1):36–38. doi: 10.1016/0014-5793(68)80094-8. [DOI] [PubMed] [Google Scholar]
- Störmer F. C. Isolation of crystalline pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. J Biol Chem. 1967 Apr 25;242(8):1756–1759. [PubMed] [Google Scholar]
- Störmer F. C. The pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. I. Kinetic studies. J Biol Chem. 1968 Jul 10;243(13):3735–3739. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B., EYRING E. J. Isoleucine and valine metabolism in Escherichia coli. IX. Utilization of acetolactate and acetohydroxybutyrate. J Biol Chem. 1960 May;235:1425–1432. [PubMed] [Google Scholar]
- Wittenberger C. L., Angelo N. Purificationa and properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Streptococcus faecalis. J Bacteriol. 1970 Mar;101(3):717–724. doi: 10.1128/jb.101.3.717-724.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


