Abstract
A mutant of yeast lacking proteinase C (carboxypeptidase Y) activity has been found by using a histochemical stain to screen mutagenized colonies. This defect segregates 2:2 in meiotic tetrads. Cell extracts lacked the esterolytic, amidase, and proteolytic activities associated with proteinase C. The absence of proteinase C does not affect mitotic growth and has no obvious effect on the formation of viable ascospores or meiotic segregation. The mutant grows on peptides known to be cleaved by proteinase C in vitro. This finding is consistent with the idea that other enzymes exist in vivo with overlapping substrate specificities.
Full text
PDF

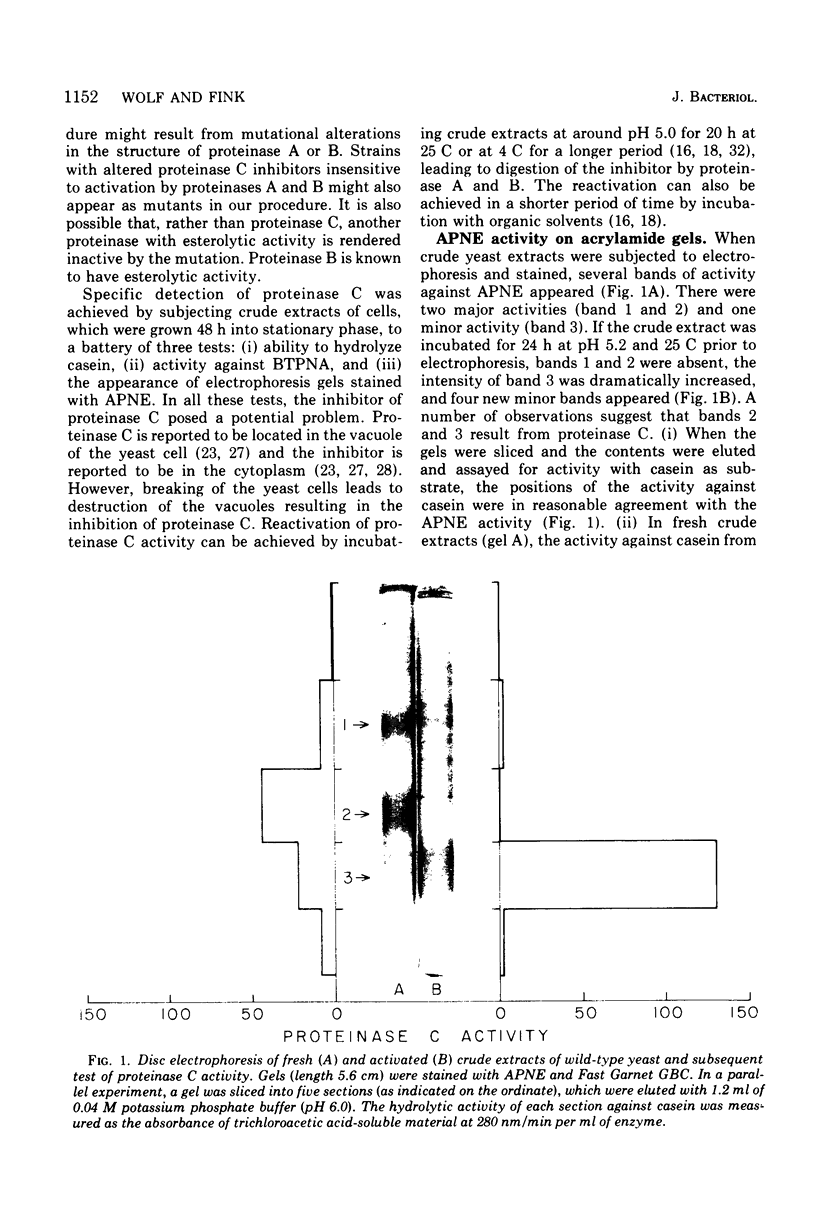

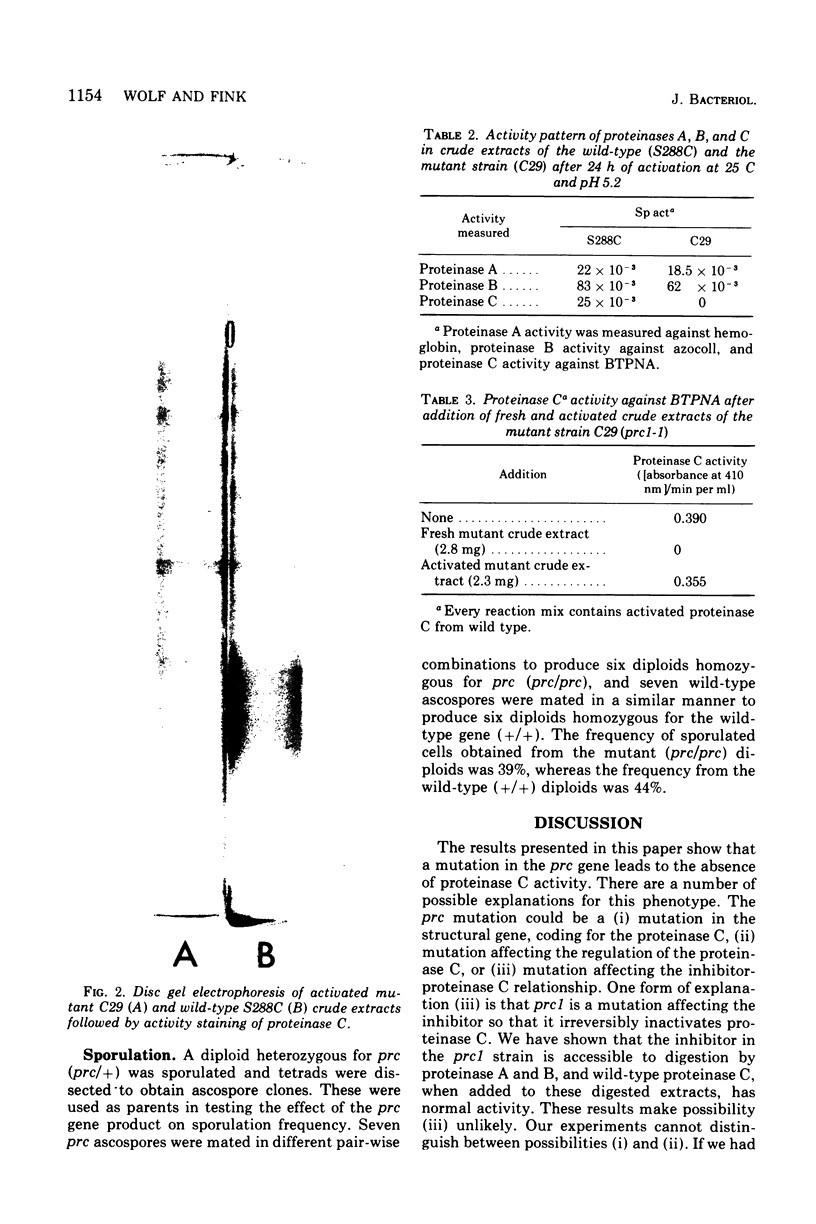


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz H., Hinze H., Holzer H. Isolation and properties of two inhibitors of proteinase B from yeast. J Biol Chem. 1974 Jul 25;249(14):4515–4521. [PubMed] [Google Scholar]
- Cabib E., Farkas V. The control of morphogenesis: an enzymatic mechanism for the initiation of septum formation in yeast. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2052–2056. doi: 10.1073/pnas.68.9.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Ulane R. Chitin synthetase activating factor from yeast, a protease. Biochem Biophys Res Commun. 1973 Jan 4;50(1):186–191. doi: 10.1016/0006-291x(73)91081-4. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R. In vivo degradation of nonsense fragments in E. coli. Nature. 1970 Dec 19;228(5277):1151–1154. doi: 10.1038/2281151a0. [DOI] [PubMed] [Google Scholar]
- HALVORSON H. Intracellular protein and nucleic acid turnover in resting yeast cells. Biochim Biophys Acta. 1958 Feb;27(2):255–266. doi: 10.1016/0006-3002(58)90332-9. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Holzer H. Participation of the tryptophan synthase inactivating system from yeast in the activation of chitin synthase. Biochem Biophys Res Commun. 1973 Jul 17;53(2):552–559. doi: 10.1016/0006-291x(73)90697-9. [DOI] [PubMed] [Google Scholar]
- Hawthorne D C, Mortimer R K. Chromosome Mapping in Saccharomyces: Centromere-Linked Genes. Genetics. 1960 Aug;45(8):1085–1110. doi: 10.1093/genetics/45.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R., Aibara S., Hata T. A unique carboxypeptidase activity of yeast proteinase C. Biochim Biophys Acta. 1970 Aug 15;212(2):359–361. doi: 10.1016/0005-2744(70)90218-4. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Moore S., Stein W. H. Carboxypeptidase from yeast. Large scale preparation and the application to COOH-terminal analysis of peptides and proteins. J Biol Chem. 1973 Apr 10;248(7):2296–2302. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Katsunuma T., Schött E., Elsässer S., Holzer H. Purification and properties of tryptophan-synthase-inactivating enzymes from yeast. Eur J Biochem. 1972 Jun 9;27(3):520–526. doi: 10.1111/j.1432-1033.1972.tb01868.x. [DOI] [PubMed] [Google Scholar]
- Kuhn R. W., Walsh K. A., Neurath H. Isolation and partial characterization of an acid carboxypeptidase from yeast. Biochemistry. 1974 Sep 10;13(19):3871–3877. doi: 10.1021/bi00716a008. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenney J. F., Dalbec J. M. Purification and properties of two proteinases from Saccharomyces cerevisiae. Arch Biochem Biophys. 1967 Apr;120(1):42–48. doi: 10.1016/0003-9861(67)90595-4. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Matile P., Wiemken A., Schellenberg M., Meyer J. Activities and cellular localization of yeast proteases and their inhibitors. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1378–1383. doi: 10.1016/0006-291x(74)90350-7. [DOI] [PubMed] [Google Scholar]
- Lodi W. R., Sonneborn D. R. Protein degradation and protease activity during the late cycle of Blastocladiella emersonii. J Bacteriol. 1974 Mar;117(3):1035–1042. doi: 10.1128/jb.117.3.1035-1042.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern H., Betz H., Holzer H. Compartmentation of inhibitors of proteinases A and B and carboxypeptidase Y in yeast. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1051–1057. doi: 10.1016/0006-291x(74)90419-7. [DOI] [PubMed] [Google Scholar]
- Matern H., Hoffmann M., Holzer H. Isolation and characterization of the carboxypeptidase Y inhibitor from yeast. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4874–4878. doi: 10.1073/pnas.71.12.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh G. L., Miller C. G. Isolation and characterization of proline peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):364–371. doi: 10.1128/jb.120.1.364-371.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouget P., Chapeville F. Leucyl-tRNA synthetase. Two forms of the enzyme: relation between structural and catalytic properties. Eur J Biochem. 1971 Dec 10;23(3):459–467. doi: 10.1111/j.1432-1033.1971.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Saheki T., Holzer H. Comparisons of the tryptophan synthase inactivating enzymes with proteinases from yeast. Eur J Biochem. 1974 Mar 1;42(2):621–626. doi: 10.1111/j.1432-1033.1974.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Saheki T., Matsuda Y., Holzer H. Urification and characterization of macromolecular inhibitors of proteinase A from yeast. Eur J Biochem. 1974 Sep 1;47(2):325–332. doi: 10.1111/j.1432-1033.1974.tb03697.x. [DOI] [PubMed] [Google Scholar]
- Takeda M., Webster R. E. Protein chain initiation and deformylation in B. subtilis homogenates. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1487–1494. doi: 10.1073/pnas.60.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. H., Hoffmann M. Tryptophan synthase from yeast. Purification by affinity chromatography, physical properties. Eur J Biochem. 1974 Jun 1;45(1):269–276. doi: 10.1111/j.1432-1033.1974.tb03551.x. [DOI] [PubMed] [Google Scholar]






