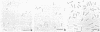Abstract
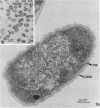
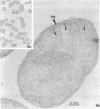
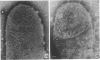
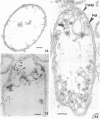
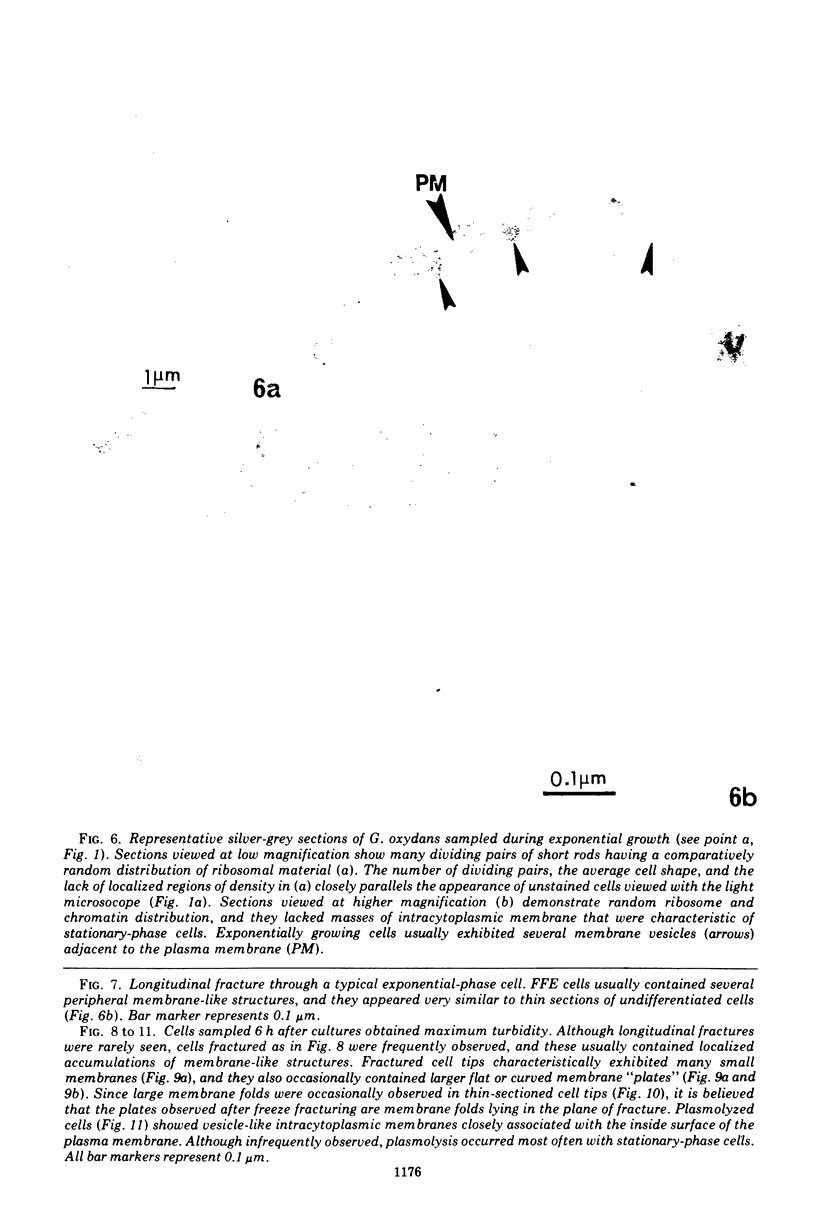
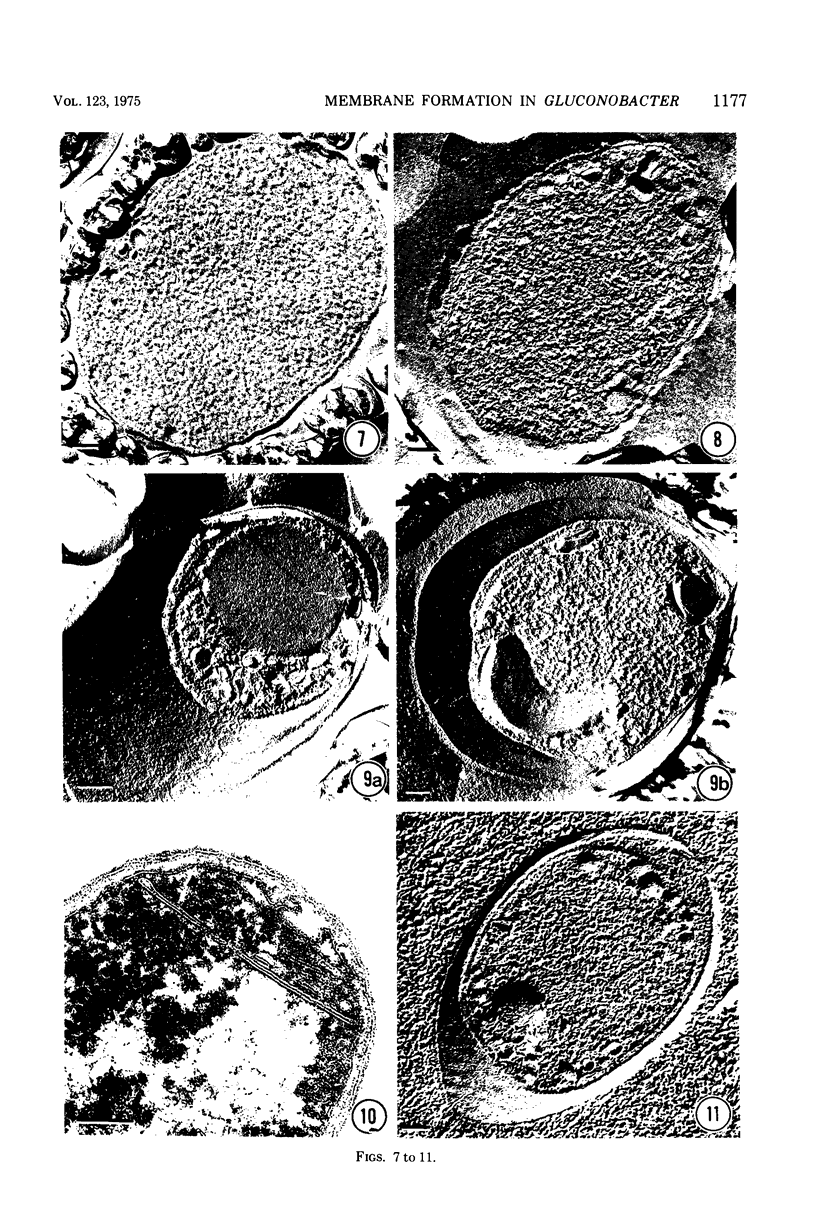
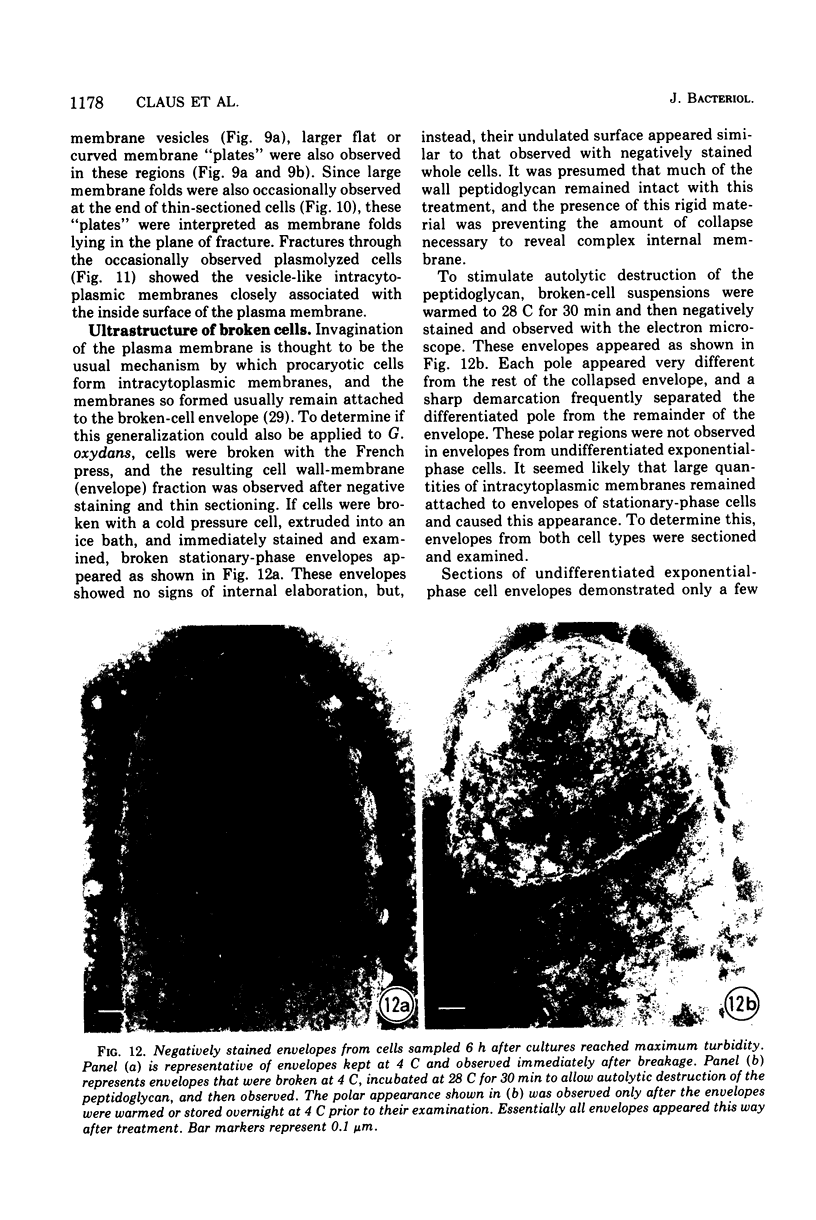
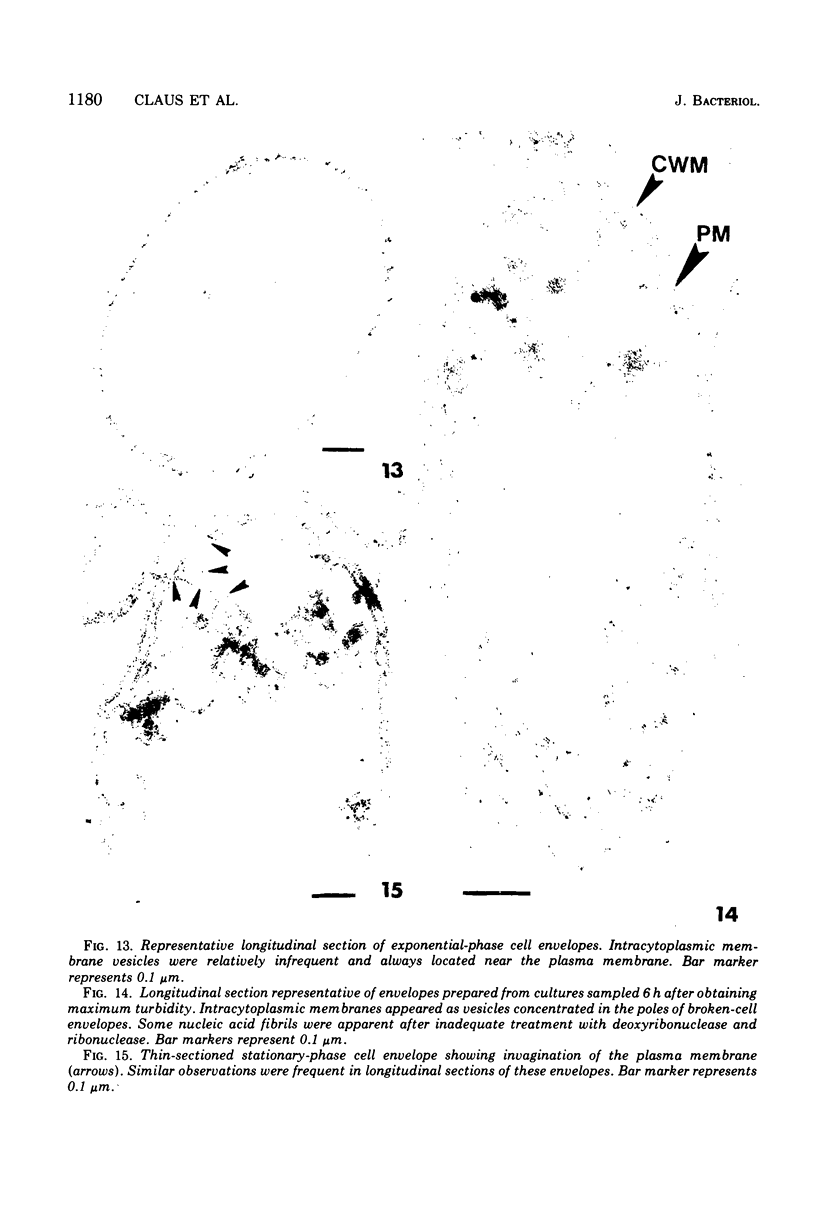
Gluconobacter oxydans is well known for the limited oxidation of compounds and rapid excretion of industrially important oxidation products. The dehydrogenases responsible for these oxidations are reportedly bound to the cell's plasma membrane. This report demonstrates that fully viable G. oxydans differentiates at the end of exponential growth by forming dense regions at the end of each cell observed with the light microscope. When these cells were thin sectioned, their polar regions contained accumulations of intracytoplasmic membranes and ribosomes not found in undifferentiated exponentially growing cells. Both freeze-fracture-etched whole cells and thin sections through broken-cell envelopes of differentiated cells demonstrate that intracytoplasmic membranes occur as a polar accumulation of vesicles that are attached to the plasma membrane. When cells were tested for the activity of the plasma membrane-associated glycerol dehydrogenase, those containing intracytoplasmic membranes were 100% more active than cells lacking these membranes. These results suggest that intracytoplasmic membranes are formed by continued plasma membrane synthesis at the end of active cell division.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASNIS R. E., BRODIE A. F. A glycerol dehydrogenase from Escherichia coli. J Biol Chem. 1953 Jul;203(1):153–159. [PubMed] [Google Scholar]
- Allen M. M. Photosynthetic membrane system in Anacystis nidulans. J Bacteriol. 1968 Sep;96(3):836–841. doi: 10.1128/jb.96.3.836-841.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. D., David G. B., Nomarski G. The zeiss-Nomarski differential interference equipment for transmitted-light microscopy. Z Wiss Mikrosk. 1969 Nov;69(4):193–221. [PubMed] [Google Scholar]
- Batzing B. L., Claus G. W. Biphasic growth of Acetobacter suboxydans on a glycerol-limiting medium. J Bacteriol. 1971 Oct;108(1):592–595. doi: 10.1128/jb.108.1.592-595.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzing B. L., Claus G. W. Fine structural changes of Acetobacter suboxydans during growth in a defined medium. J Bacteriol. 1973 Mar;113(3):1455–1461. doi: 10.1128/jb.113.3.1455-1461.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belly R. T., Claus G. W. Effect of amino acids on the growth of Acetobacter suboxydans. Arch Mikrobiol. 1972;83(3):237–245. doi: 10.1007/BF00645124. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R., Poindexter J. S. The internal membranes of Caulobacter crescentus. J Gen Microbiol. 1966 Feb;42(2):301–308. doi: 10.1099/00221287-42-2-301. [DOI] [PubMed] [Google Scholar]
- Cota-Robles E. H. Internal membranes in cells of Escherichia coli. J Ultrastruct Res. 1966 Dec;16(5):626–639. doi: 10.1016/s0022-5320(66)80010-2. [DOI] [PubMed] [Google Scholar]
- DELEY J., KERSTERS K. OXIDATION OF ALIPHATIC GLYCOLS BY ACETIC ACID BACTERIA. Bacteriol Rev. 1964 Jun;28:164–180. [PMC free article] [PubMed] [Google Scholar]
- Davies S. L., Whittenbury R. Fine structure of methane and other hydrocarbon-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):227–232. doi: 10.1099/00221287-61-2-227. [DOI] [PubMed] [Google Scholar]
- GEITLER L. Lamelläre Struktur des Chromatoplasmas von Cyanophyceen in mikroskopischen Dimensionen und Baueigentümlichkeiten des Protoplasten von Chroococcus turgidus. Arch Mikrobiol. 1958;29(2):179–188. [PubMed] [Google Scholar]
- Greenfield S., Claus G. W. Nonfunctional tricarboxylic acid cycle and the mechanism of glutamate biosynthesis in Acetobacter suboxydans. J Bacteriol. 1972 Dec;112(3):1295–1301. doi: 10.1128/jb.112.3.1295-1301.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchins A. D., Kahn A. J., Slepecky R. A. Interference contrast and phase contrast microscopy of sporulation and germination of Bacillus megaterium. J Bacteriol. 1968 Nov;96(5):1811–1817. doi: 10.1128/jb.96.5.1811-1817.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Conti S. F., Fuller R. C. Effect of light intensity on the formation of the photochemical apparatus in the green bacterium Chloropseudomonas ethylicum. J Bacteriol. 1966 Jan;91(1):349–355. doi: 10.1128/jb.91.1.349-355.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERSTERS K., WOOD W. A., DELEY J. POLYOL DEHYDROGENASES OF GLUCONOBACTER OXYDANS. J Biol Chem. 1965 Mar;240:965–974. [PubMed] [Google Scholar]
- KLUNGSOYR L., KING T. E., CHELDELIN V. H. Oxidative phosphorylation in Acetobacter suboxydans. J Biol Chem. 1957 Jul;227(1):135–149. [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Oppenheim J., Marcus L. Correlation of ultrastructure in Azotobacter vinelandii with nitrogen source for growth. J Bacteriol. 1970 Jan;101(1):286–291. doi: 10.1128/jb.101.1.286-291.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Shah V. K., Brill W. J. Internal membrane control in Azotobacter vinelandii. J Bacteriol. 1973 Jun;114(3):1346–1350. doi: 10.1128/jb.114.3.1346-1350.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt T. E., Cole G. C., Bland J., Hanson R. S. Isolation and characterization of bacteria that grow on methane and organic compounds as sole sources of carbon and energy. J Bacteriol. 1974 Nov;120(2):955–964. doi: 10.1128/jb.120.2.955-964.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope L. M., Hoare D. S., Smith A. J. Ultrastructure of Nitrobacter agilis grown under autotrophic and heterotrophic conditions. J Bacteriol. 1969 Feb;97(2):936–939. doi: 10.1128/jb.97.2.936-939.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W., Waterbury J. B., Trüper H. G. Fine structure of Ectothiorhodospira mobilis Pelsh. J Bacteriol. 1968 Jun;95(6):2374–2392. doi: 10.1128/jb.95.6.2374-2392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- WIDMER C., KING T. E., CHELDELIN V. H. Particulate oxidase systems in Acetobacter suboxydans. J Bacteriol. 1956 Jun;71(6):737–737. doi: 10.1128/jb.71.6.737-737.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Mandel M. Comparison of the morphology and deoxyribonucleic acid composition of 27 strains of nitrifying bacteria. J Bacteriol. 1971 Aug;107(2):563–569. doi: 10.1128/jb.107.2.563-569.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver T. L., Dugan P. R. Ultrastruct of Methylosinus trichosporium as revealed by freeze etching. J Bacteriol. 1975 Feb;121(2):704–710. doi: 10.1128/jb.121.2.704-710.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum G., Fischman D. A., Okuda S. Membrane modifications in nutritionally induced filamentous Escherichia coli B. J Cell Biol. 1970 Jun;45(3):493–508. doi: 10.1083/jcb.45.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]