Abstract
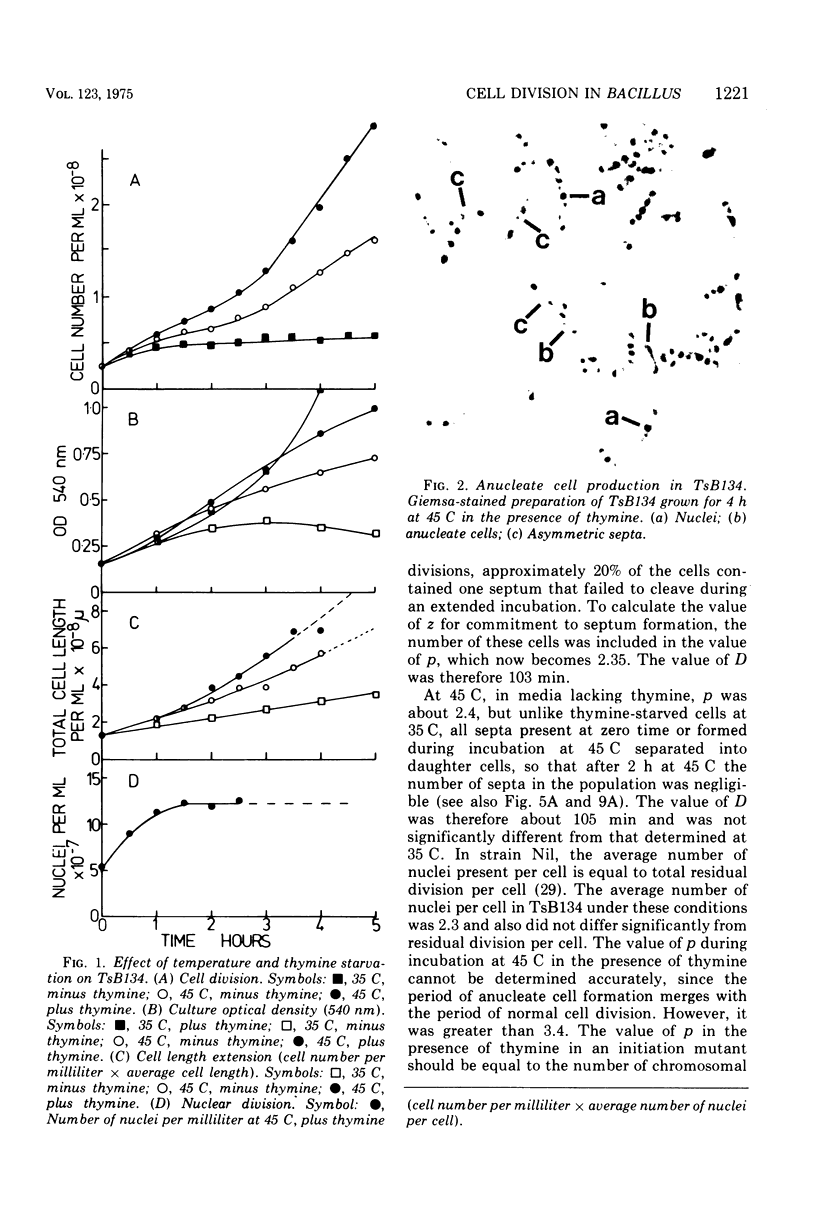
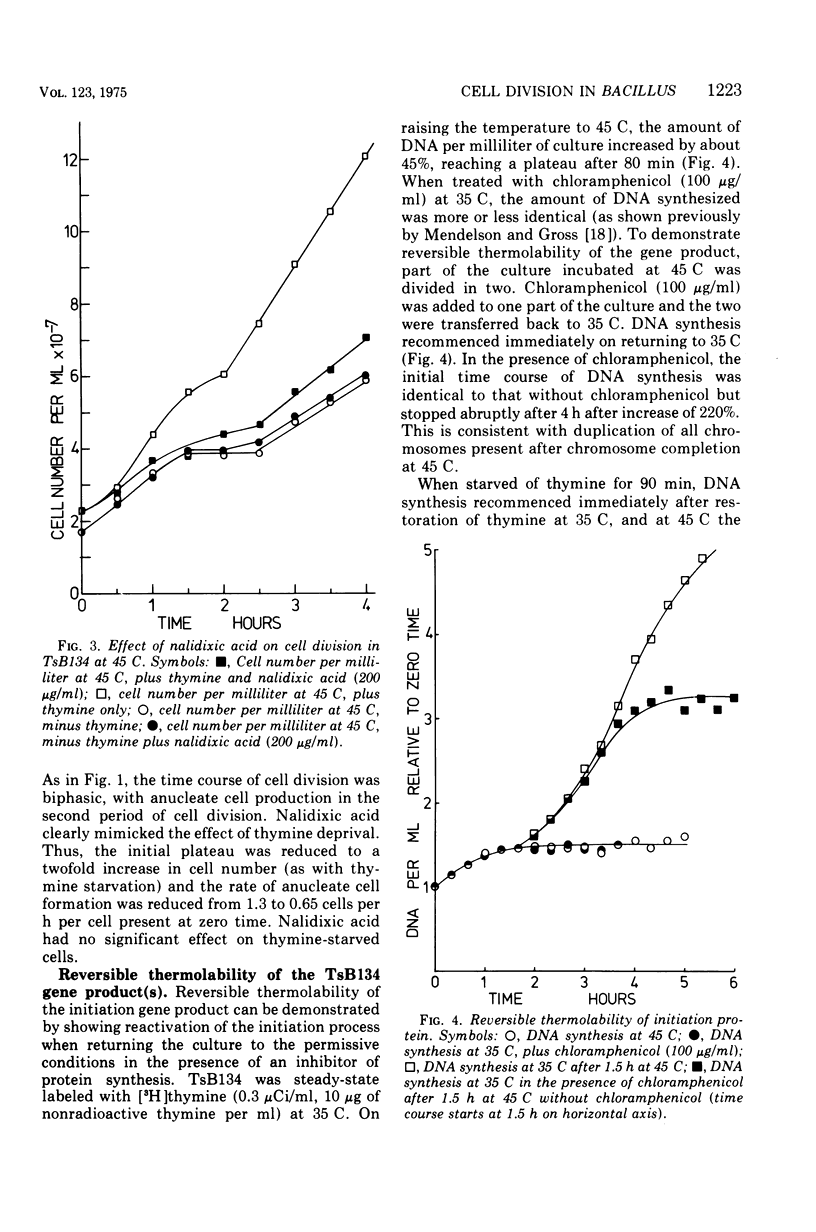
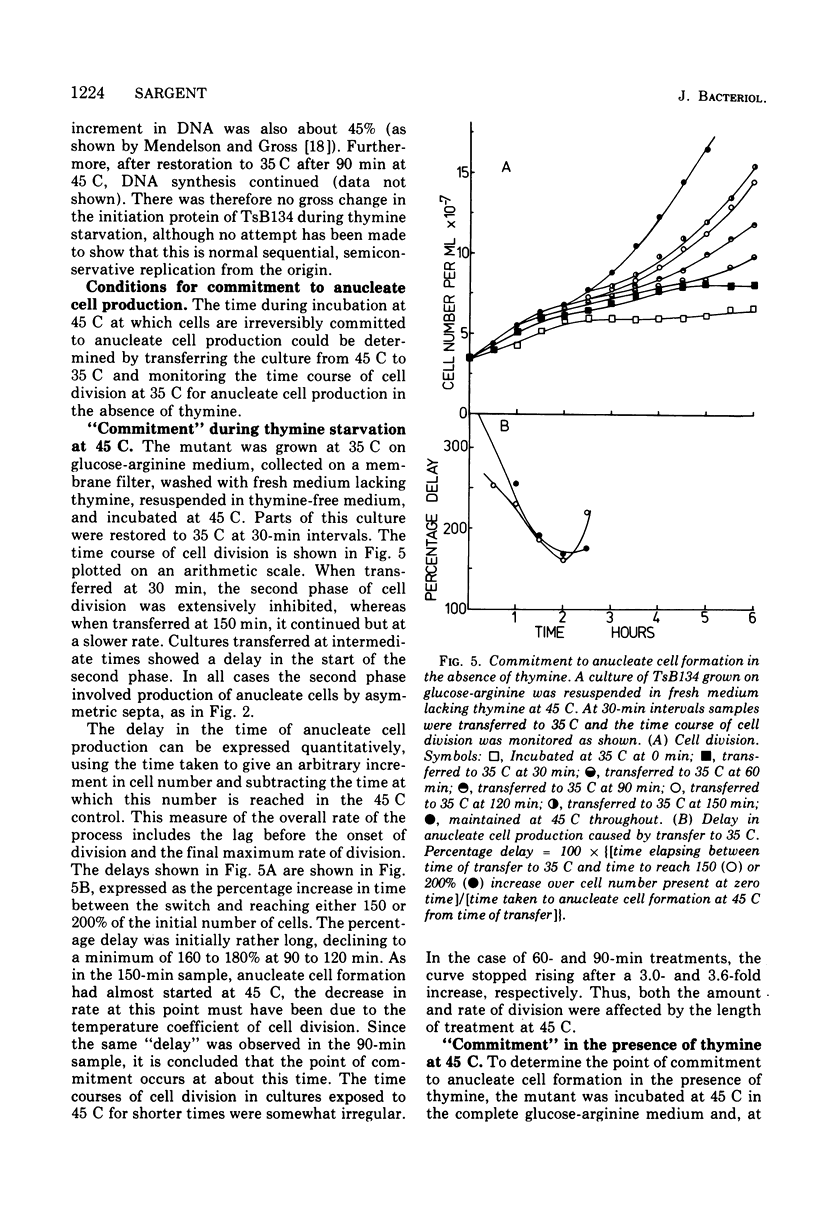
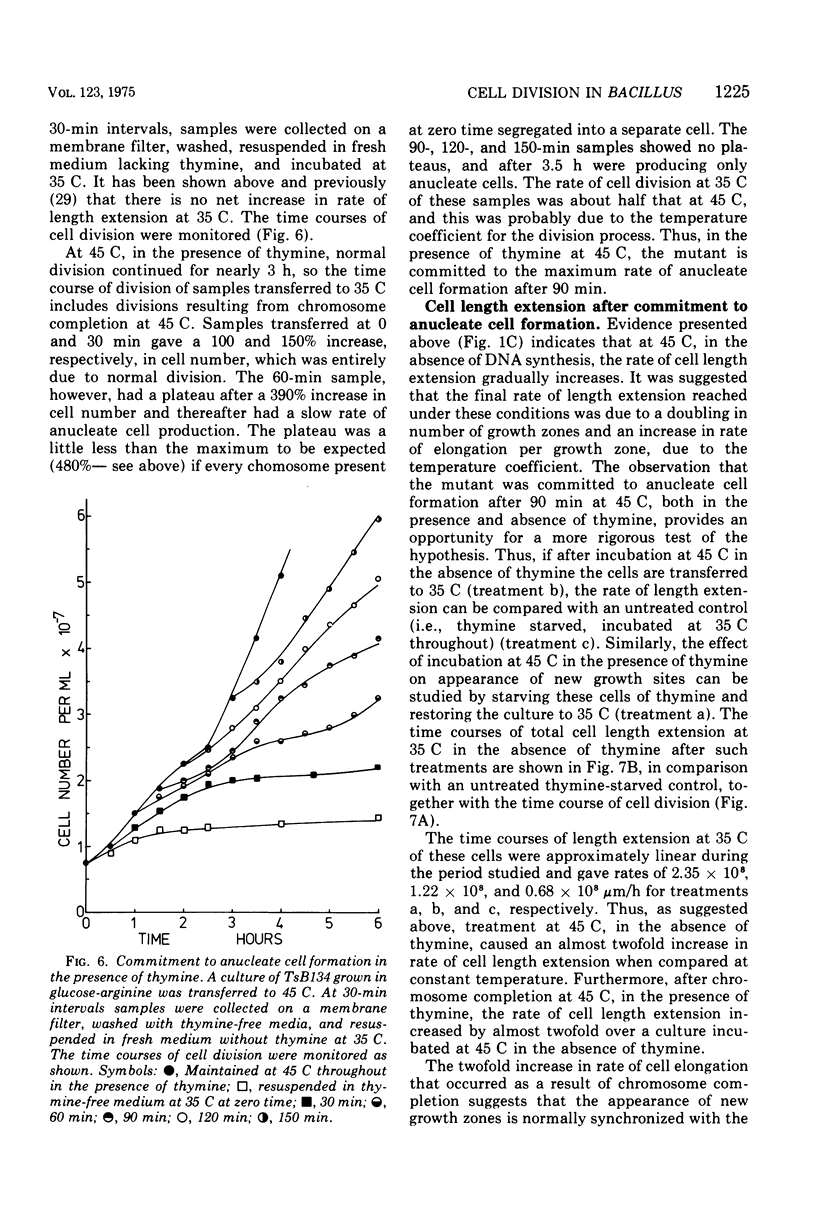
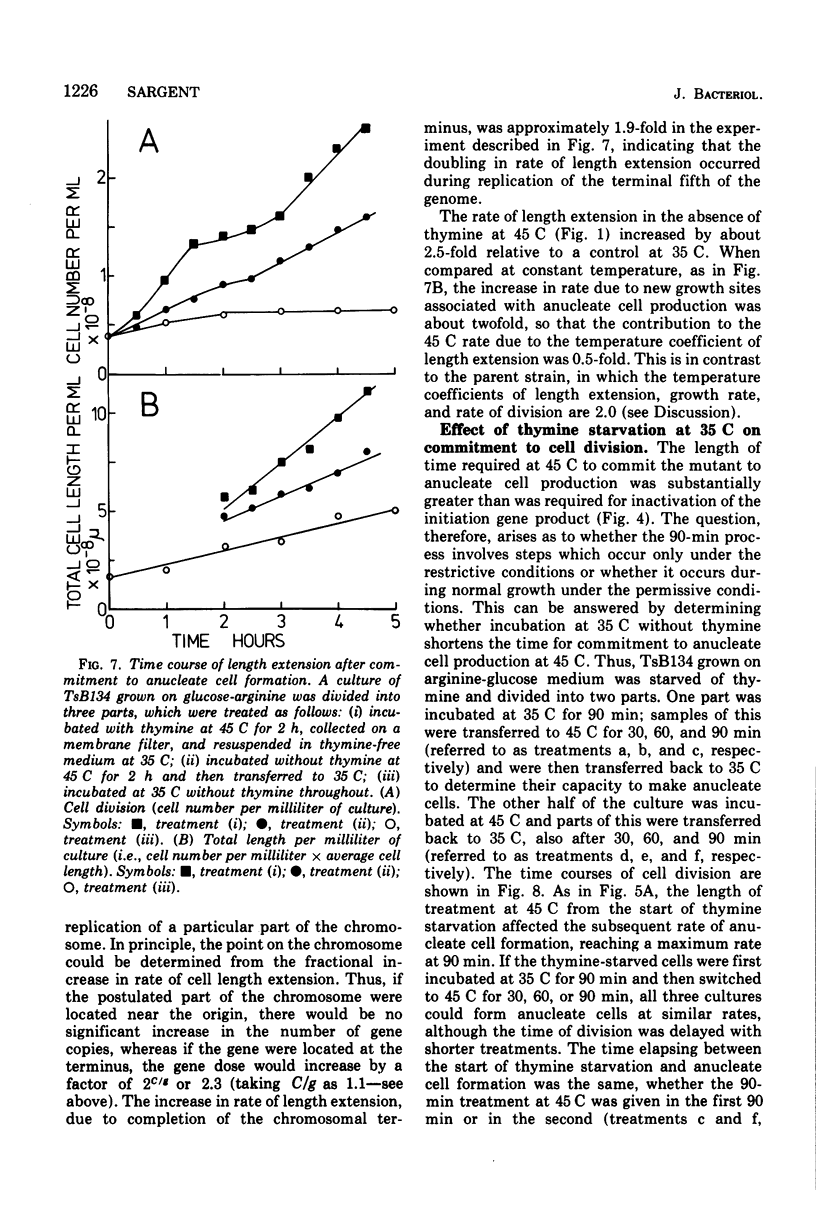
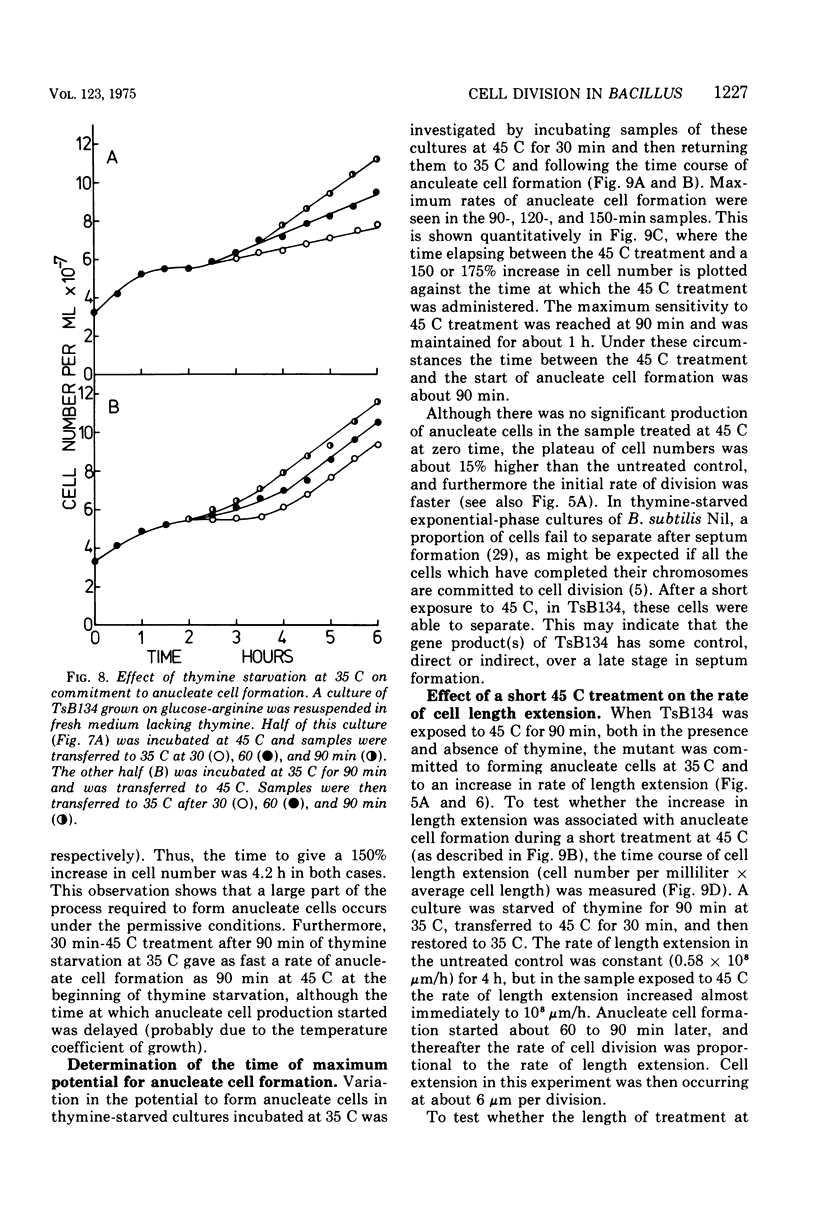
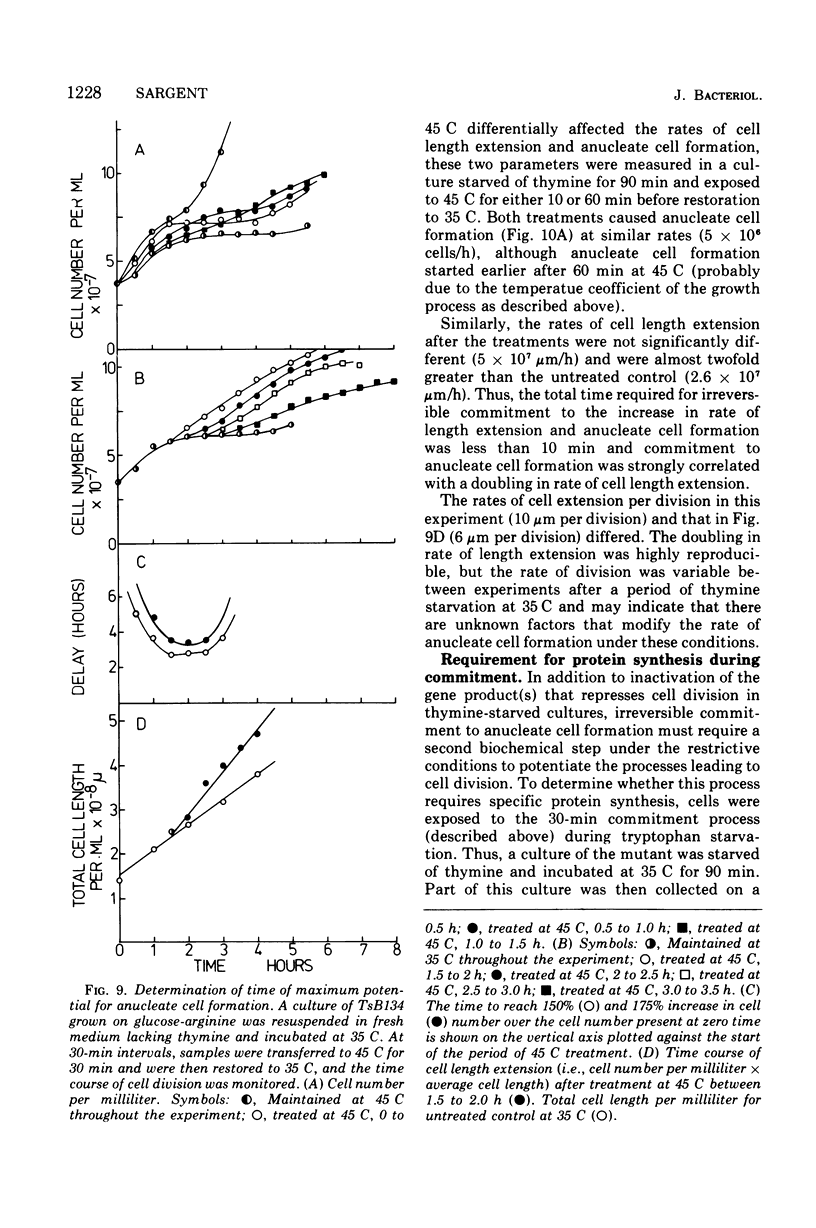
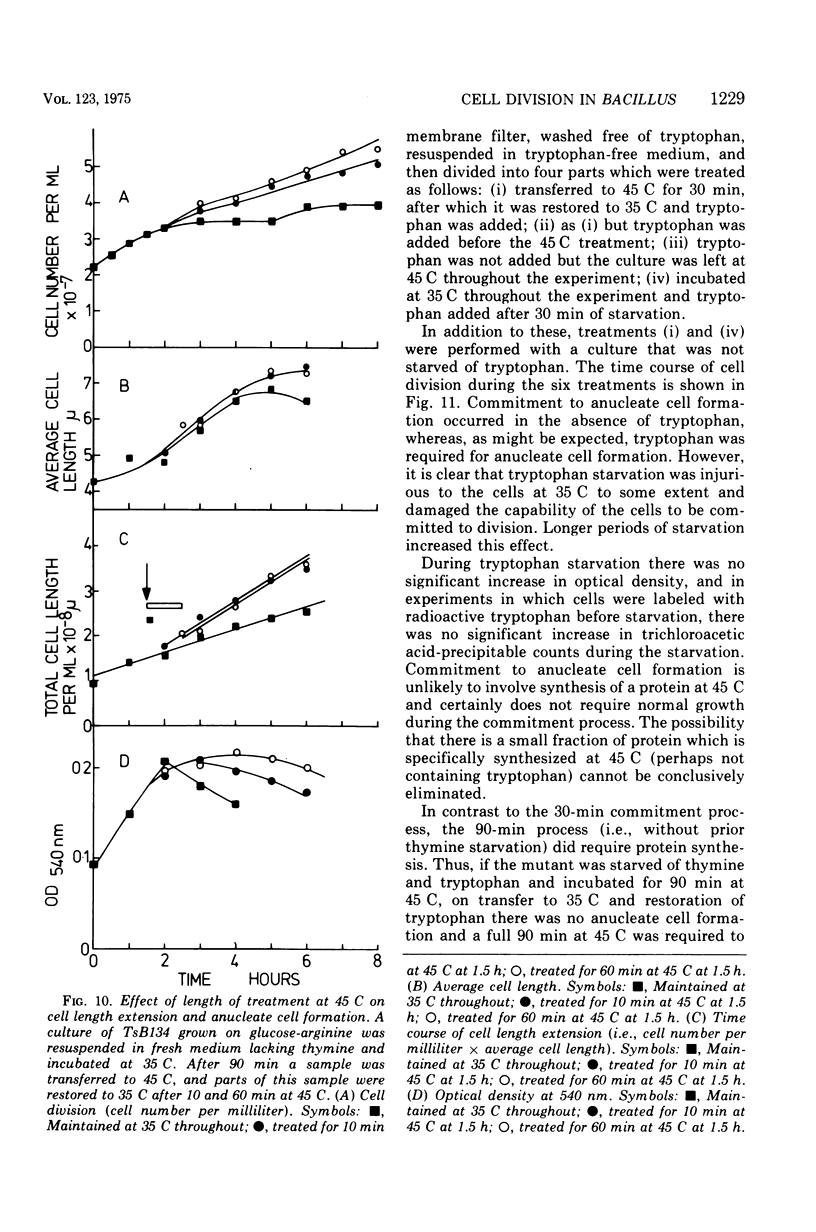
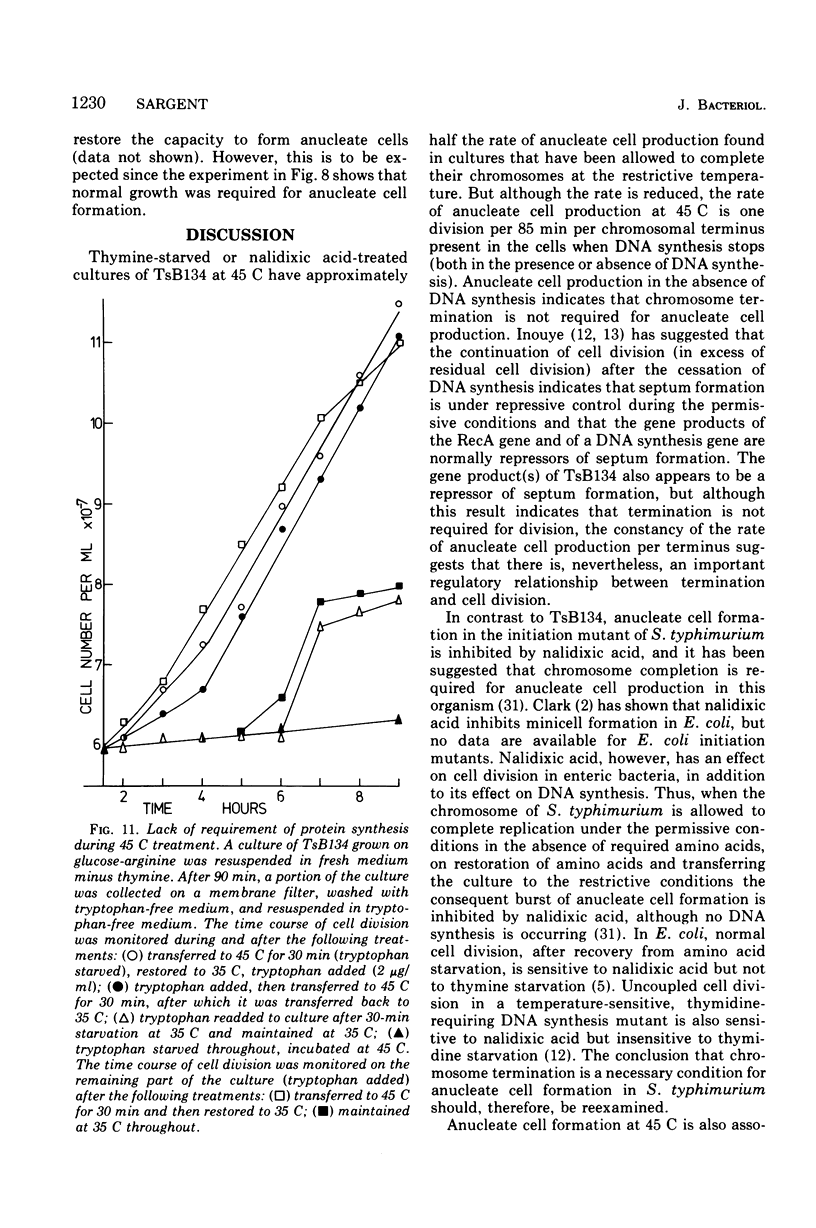
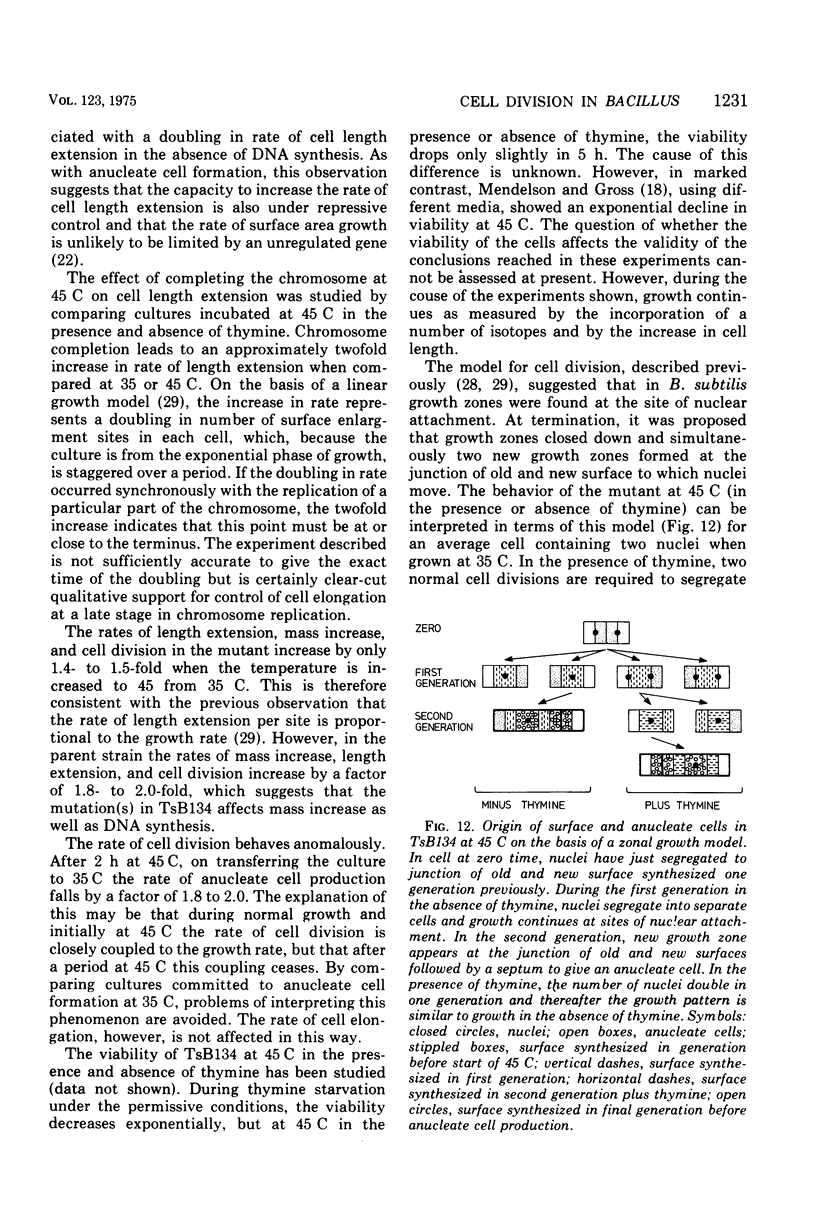
At 45 C, in a temperature-sensitive initiation mutant (TsB134) of Bacillus subtilis 168 Thy- tryp-, growing in a glucose-arginine minimal medium, chromosome completion occurred over a period of 80 to 90 min, after which there was no further nuclear division. Normal symmetrical cell divisions continued for a generation afterwards, so that nuclei were segregated into separate cells. During this period asymmetric divisions started to occur. Septa appeared at 25 to 30% from one end of the cell, giving a small anucleate cell and a larger nucleate cell. During inhibition of deoxyribonucleic acid (DNA) synthesis by thymine starvation under the restrictive conditions, asymmetrical division also occurred until there was approximately one nucleus per cell (about one generation time). Asymmetric division, giving anucleate cells, then occurred. Similar results were obtained when DNA synthesis was inhibited by nalidixic acid. After 3 h at 45 C, the rate of anucleate cell production in the presence and absence of thymine was constant at one division per 85 min per chromosome terminus present when DNA synthesis stopped. In the absence of DNA synthesis (during thymine starvation) at 35 C, growth in cell length was linear (i.e., the rate was constant), but at 45 C during thymine starvation the rate gradually increased by more than twofold. It is suggested that this was due to the establishment of new sites of growth associated with anucleate cell production. In the presence of thymine at 45 C, the rate of length extension increased by more than fourfold, which it is suggested was caused by the appearance of new growth zones as a result of chromosome termination and a contribution associated with anucleate cell production. If the mutant was incubated at 45 C for 90 min, both in the presence and absence of thymine, then anucleate cell formation could continue on restoration to 35 C in the absence of thymine...
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. M., Mavis R. D., Vagelos P. R. Altered phospholipid metabolism in a temperature-sensitive mutant of Escherichia coli, CR 34 T 46 . Biochim Biophys Acta. 1972 Aug 11;270(4):504–512. [PubMed] [Google Scholar]
- Clark D. J. Regulation of deoxyribonucleic acid replication and cell division in Escherichia coli B-r. J Bacteriol. 1968 Oct;96(4):1214–1224. doi: 10.1128/jb.96.4.1214-1224.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Coyne S. I., Mendelson N. H. Clonal analysis of cell division in the Bacillus subtilis div IV-B1 minicell-producing mutant. J Bacteriol. 1974 Apr;118(1):15–20. doi: 10.1128/jb.118.1.15-20.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix D. E., Helmstetter C. E. Coupling between chromosome completion and cell division in Escherichia coli. J Bacteriol. 1973 Sep;115(3):786–795. doi: 10.1128/jb.115.3.786-795.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Growth of the bacterial cell. Nature. 1970 Sep 19;227(5264):1220–1224. doi: 10.1038/2271220a0. [DOI] [PubMed] [Google Scholar]
- Donachie W. D. Control of cell division in Escherichia coli: experiments with thymine starvation. J Bacteriol. 1969 Oct;100(1):260–268. doi: 10.1128/jb.100.1.260-268.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. D., Karamata D., Hempstead P. G. Temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Cold Spring Harb Symp Quant Biol. 1968;33:307–312. doi: 10.1101/sqb.1968.033.01.034. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E., Pierucci O. Cell division during inhibition of deoxyribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1968 May;95(5):1627–1633. doi: 10.1128/jb.95.5.1627-1633.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Jacob F., Ryter A., Buttin G., Nakai T. On the process of cellular division in Escherichia coli. I. Asymmetrical cell division and production of deoxyribonucleic acid-less bacteria. J Mol Biol. 1968 Jul 14;35(1):175–192. doi: 10.1016/s0022-2836(68)80046-4. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Inouye M. Pleiotropic effect of the rec A gene of Escherichia coli: uncoupling of cell division from deoxyribonucleic acid replication. J Bacteriol. 1971 May;106(2):539–542. doi: 10.1128/jb.106.2.539-542.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Unlinking of cell division from deoxyribonucleic acid replication in a temperature-sensitive deoxyribonucleic acid synthesis mutant of Escherichia coli. J Bacteriol. 1969 Sep;99(3):842–850. doi: 10.1128/jb.99.3.842-850.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Donachie W. D. Chromosome replication, transcription and control of cell division in Escherichia coli. Nat New Biol. 1973 May 23;243(125):100–103. [PubMed] [Google Scholar]
- Karamata D., Gross J. D. Isolation and genetic analysis of temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Mol Gen Genet. 1970;108(3):277–287. doi: 10.1007/BF00283358. [DOI] [PubMed] [Google Scholar]
- Kennett R. H., Sueoka N. Gene expression during outgrowth of Bacillus subtilis spores. The relationship between gene order on the chromosome and temporal sequence of enzyme synthesis. J Mol Biol. 1971 Aug 28;60(1):31–44. doi: 10.1016/0022-2836(71)90445-1. [DOI] [PubMed] [Google Scholar]
- Mendelson N. H., Gross J. D. Characterization of a temperature-sensitive mutant of Bacillus subtilis defective in deoxyribonucleic acid replication. J Bacteriol. 1967 Nov;94(5):1603–1608. doi: 10.1128/jb.94.5.1603-1608.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peypoux F., Michel G. Etude des phospholipides d'une souche sauvage et d'une souche mutante thermosensible d'Escherichia coli. Biochim Biophys Acta. 1970 Dec 15;218(3):453–462. [PubMed] [Google Scholar]
- Pierucci O., Helmstetter C. E. Chromosome replication, protein synthesis and cell division in Escherichia coli. Fed Proc. 1969 Nov-Dec;28(6):1755–1760. [PubMed] [Google Scholar]
- Previc E. P. Biochemical determination of bacterial morphology and the geometry of cell division. J Theor Biol. 1970 Jun;27(3):471–497. doi: 10.1016/s0022-5193(70)80010-8. [DOI] [PubMed] [Google Scholar]
- Pritchard R. H. Review lecture on the growth and form of a bacterial cell. Philos Trans R Soc Lond B Biol Sci. 1974 Feb 21;267(886):303–336. doi: 10.1098/rstb.1974.0003. [DOI] [PubMed] [Google Scholar]
- Pritchard R. H., Zaritsky A. Effect of thymine concentration on the replication velocity of DNA in a thymineless mutant of Escherichia coli. Nature. 1970 Apr 11;226(5241):126–131. doi: 10.1038/226126a0. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H., Coyne S. I., Hallock L. L., Cole R. M. Minicells of Bacillus subtilis. J Bacteriol. 1973 May;114(2):860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUD I. J., SCHAECHTER M. DEPENDENCE OF THE CONTENT OF CELL ENVELOPES ON THE GROWTH RATE OF BACILLUS MEGATERIUM. J Bacteriol. 1964 Dec;88:1612–1617. doi: 10.1128/jb.88.6.1612-1617.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Control of cell length in Bacillus subtilis. J Bacteriol. 1975 Jul;123(1):7–19. doi: 10.1128/jb.123.1.7-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Membrane synthesis in synchronous cultures of Bacillus subtilis 168. J Bacteriol. 1973 Oct;116(1):397–409. doi: 10.1128/jb.116.1.397-409.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Nuclear segregation in Bacillus subtilis. Nature. 1974 Jul 19;250(463):252–254. doi: 10.1038/250252a0. [DOI] [PubMed] [Google Scholar]
- Sargent M. G. Synchronous cultures of Bacillus subtilis obtained by filtration with glass fiber filters. J Bacteriol. 1973 Nov;116(2):736–740. doi: 10.1128/jb.116.2.736-740.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K. P., Rowbury R. J. Alteration of the rate of cell division independent of the rate of DNA synthesis in a mutant of Salmonella typhimurium. Mol Gen Genet. 1972;115(2):122–125. doi: 10.1007/BF00277291. [DOI] [PubMed] [Google Scholar]
- Shannon K. P., Spratt B. G., Rowbury R. J. Cell division and the production of cells lacking nuclear bodies in a mutant of Salmonella typhimurium. Mol Gen Genet. 1972;118(2):185–197. doi: 10.1007/BF00267087. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Rowbury R. J. A mutant in the initiation of DNA synthesis in Salmonella typhimurium. J Gen Microbiol. 1970 Dec;64(2):127–138. doi: 10.1099/00221287-64-2-127. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Rowbury R. J. Cell division in a mutant of Salmonella typhimurium which is temperature-sensitive for DNA synthesis. J Gen Microbiol. 1971 Mar;65(3):305–314. doi: 10.1099/00221287-65-3-305. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Rowbury R. J. Physiological and genetical studies on a mutant of Salmonella typhimurium which is temperature-sensitive for DNA synthesis. Mol Gen Genet. 1972;114(1):35–49. doi: 10.1007/BF00268745. [DOI] [PubMed] [Google Scholar]
- Steinberg W., Halvorson H. O. Timing of enzyme synthesis during outgrowth of spores of Bacillus cereus. I. Ordered enzyme synthesis. J Bacteriol. 1968 Feb;95(2):469–478. doi: 10.1128/jb.95.2.469-478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Collins J. F., Donachie W. D. Quantal behavior of a diffusible factor which initiates septum formation at potential division sites in Escherichia coli. J Bacteriol. 1974 May;118(2):407–413. doi: 10.1128/jb.118.2.407-413.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Changes in cell size and shape associated with changes in the replication time of the chromosome of Escherichia coli. J Bacteriol. 1973 May;114(2):824–837. doi: 10.1128/jb.114.2.824-837.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]