Abstract
Human serum albumin (HSA) derivatized with cis-aconitic anhydride was covalently coupled to liposomes with a size of approximately 100 nm [polyaconitylated HSA (Aco-HSA) liposomes]. Within 30 min after injection into a rat, Aco-HSA liposomes were completely cleared from the blood and almost exclusively taken up by the liver, whereas in control liposomes 80% was still present in the blood at that time. Endothelial cells were shown to account for almost two-thirds of the hepatic uptake of the Aco-HSA liposomes, the remainder being recovered mainly in the liver macrophages (Kupffer cells). With fluorescently labeled liposomes it was shown that the Aco-HSA liposomes target a vast majority (>85%) of the cells in the endothelial cell population. Control liposomes were not taken up to a significant extent by the endothelial cells. Uptake of Aco-HSA liposomes by both endothelial and Kupffer cells was inhibited by preinjection with polyinosinic acid, indicating the involvement of scavenger receptors in the uptake process. The uptake of Aco-HSA liposomes by liver endothelial cells was dependent on liposome size; with increasing liposome diameter endothelial cell uptake decreased in favor of Kupffer cell uptake. We have demonstrated that massive in vivo targeting of liposomes to a defined cell population other than macrophages is possible. Aco-HSA liposomes thus may represent an attractive drug carrier system for treatment of various liver or liver endothelium-associated disorders.
Over the past 10 years the number of reports on nonviral, often lipid-based, gene and antisense oligonucleotide delivery systems have steadily increased (1, 2). The in vitro transfection efficiencies of such systems are approaching that of viral systems without sharing some of the drawbacks of the latter. The ultimate goal for such systems is to apply them in vivo. However, relatively few in vivo experiments, in which such delivery systems are administered intravenously, have been reported thus far (3, 4). A general aim is to manipulate the functions of a specific cell population, e.g., tumor cells, and to deliver the particular material preferentially or even exclusively at the target site. Site-specific delivery still constitutes a formidable task, as long as cells other than macrophages are the target. Macrophages, mainly in liver and spleen, are the principal site of uptake of systemically circulating particles such as liposomes and nanoparticles, despite extensive efforts to increase their systemic circulation time. So-called “long circulating” liposomes have been developed by incorporating surface-bound polyethylene glycol or other hydrophilic moieties (5). Attempts to actively target liposomes to tumors thus far have met with limited success. Significant tumor accumulation of liposomes relative to surrounding tissue has been achieved. However, at best only minor proportions of the injected dose actually concentrate in solid tumors, whereas the major fraction invariably ends up in macrophages of liver and spleen. This is due not only to the competitive phagocytic potential of these cells but also to poor extravasation of a particulate carrier. With respect to accessibility, the vascular endothelium would seem to be an ideal target cell population for drug carriers, and attempts to achieve such targeting, preferably in an organ-specific way, have been described (6, 7). In the present study we report on the massive targeting of an i.v.-administered dose of liposomes to a single endothelial cell population: the sinusoidal endothelial cells in the liver. Recently, we showed that liposomes that were surface-modified by covalent coupling of polyaconitylated human serum albumin (Aco-HSA) (8) display an intrinsic anti-HIV type-1 activity (9). These polyanionized proteoliposomes were cleared within 30 min from the general circulation after i.v. administration to rats. It was shown that this rapid clearance was mediated by the coupled protein and that the liposomes were present almost exclusively in the liver. The polyanionic nature of the Aco-HSA suggested the role of scavenger receptors on sinusoidal cell types in this process. These receptors have been implicated in the uptake from the blood of polyanionic macromolecules like acetylated low-density lipoprotein, oxidatively modified low-density lipoprotein, and various modified albumins (10, 11).
Therefore, we set out to determine which cell types in the liver are responsible for the abundant uptake of Aco-HSA liposomes, and we investigated the effects of the scavenger receptor inhibitor polyinosinic acid as well as the size of the liposomes on the tissue and intrahepatic distribution.
MATERIALS AND METHODS
Materials.
Phospholipids were from Avanti Polar Lipids. Cholesterol, N-succinimidyl-S-acetylthioacetate, and cis-aconitic anhydride were from Sigma. [3H]cholesteryl oleyl ether was purchased from Amersham. Pronase (from Streptomyces griseus) and collagenase type A were from Boehringer Mannheim. 1,1′dioctadecyl-3,3,3′,3′-tetramethyl indocarbocyanine perchlorate (DiI) was purchased from Molecular Probes. All other chemicals were analytical grade or the best grade available.
Liposome Preparation.
Liposomes were prepared as follows. Lipids from stock solutions of egg phosphatidylcholine, cholesterol, and maleimido-4-(p-phenylbutyryl)-phosphatidylethanolamine in chloroform/methanol (9:1) were mixed in a molar ratio of 23:16:1, dried under reduced nitrogen pressure, dissolved in cyclohexane, and lyophilized. A trace amount of [3H]cholesteryl oleyl ether was added to each preparation as a nondegradable marker. In some experiments 1 mol% DiI was added to the lipid mixture as a fluorescent marker. The liposomes then were hydrated in buffer [10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Hepes)/135 mM NaCl, pH 6.7] and sized by repeated extrusion (13 times) through polycarbonate filters with a pore size of 50 nm (Costar). In some experiments filters with a pore size of 100, 200, or 400 nm were used, as indicated. Preparation of HSA in which an average of 53 of the 60 available ɛ amino groups were derivatized with cis-aconitic anhydride and coupling of the protein to the liposomes have been described earlier in detail (9). The Aco-HSA liposomes were characterized by determining protein (12) and phospholipid phosphorus content (13) and by particle size analysis by dynamic light scattering using a Nicomp model 370 submicron particle analyzer (NICOMP particle sizing systems, Santa Barbara, CA). Unless stated otherwise the liposomes used in the experiments had a mean diameter of 74.0 ± 1.9 nm for control liposomes (i.e., without protein coupled) and 92.1 ± 10.2 nm for Aco-HSA liposomes. Aco-HSA liposomes contained 33.5 ± 1.8 mg of protein per mmol of total lipid. Control liposomes were prepared from the same lipid mixture as used for the preparation of Aco-HSA liposomes, but instead of coupling Aco-HSA, reactive maleimido groups on the liposome surface were allowed to react with cysteine (9).
Tissue Distribution Studies.
For determination of tissue distribution, 1 μmol (total lipid) per 100 g of body weight of radiolabeled liposomes was injected into male Wag/Rij rats (200–250 g) via the penile vein under pentobarbital anesthesia. At the indicated time, a blood sample was taken, and tissues were removed and processed for measurement of radioactivity as described before (9). The total amount of radioactivity in the serum was calculated using the equation: serum volume (ml) = [0.0219 × body weight (g)] + 2.66 (14). When appropriate, rats were injected 1 min before liposome injection with 5 mg polyinosinic acid per 200 g of body weight.
Isolation of Liver Cells.
Liver endothelial cells and Kupffer cells were isolated after pronase perfusion of the organ, followed by centrifugation and counterflow centrifugal elutriation as described (15). Liver parenchymal cells were isolated after collagenase perfusion as described (16). The numbers of cells of the different fractions were determined microscopically. Liposome uptake by the different cell types was determined by measurement of the cell associated radioactivity. In the case of parenchymal cell uptake of liposomes the data were corrected for Kupffer cell contamination (maximally 2%) (17). Uptake of fluorescent (DiI) liposomes was determined microscopically using an Olympus AX True Research System Microscope model AX70 equipped with a standard rhodamine emission and exitation combination. Uptake of fluorescently labeled liposomes by endothelial and Kupffer cells also was determined by flow cytometry performed on an Epics-Elite flow cytometer (Coulter) equipped with an argon laser operating at 15 mW and 488 nm. The filter setting for measurement of DiI fluorescence was 525 band pass. Measurements were performed on 10,000 events, and forward scatter and side scatter were used to gate living cells. Data were analyzed using standard elite software.
Statistical Evaluation.
Statistical significance of differences was evaluated by a two-tailed unpaired Student’s t test.
RESULTS
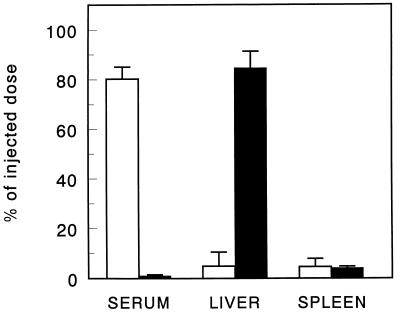
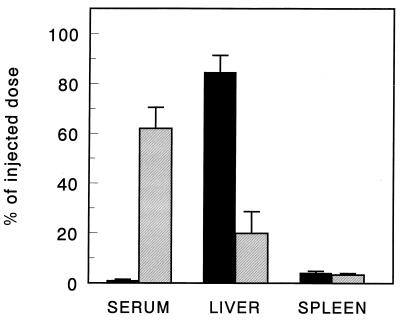
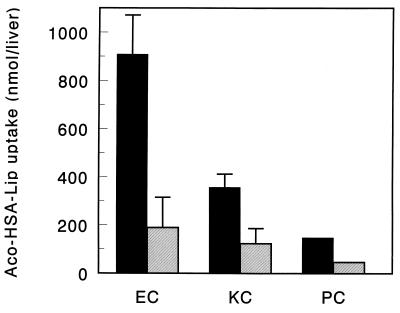
The organ distribution of Aco-HSA liposomes and control liposomes 30 min after injection into rats is shown in Fig. 1. At 30 min after injection, the Aco-HSA liposomes were completely cleared from the blood circulation, as opposed to only 20% of the control liposomes. The rapid clearance of Aco-HSA liposomes was almost entirely accounted for by uptake into the liver, the spleen being the only other organ in which a small, but significant, amount of liposomal label was found. The small fraction of the control liposomes, cleared from the circulation after 30 min, equally distributed between liver and spleen. Injection of polyinosinic acid, 1 min before injection with liposomes, reduced the liver uptake of Aco-HSA liposomes to 24% of the value obtained in untreated rats (Fig. 2). Enzymatic digestion of the liver and the subsequent separation and isolation of liver endothelial cells and Kupffer cells showed that the endothelial cells and, to a lesser extent, Kupffer cells accounted for 90% of the total hepatic uptake of Aco-HSA liposomes (Fig. 3). This uptake represents only Aco-HSA liposomes internalized by the cells, because ectoproteins, including membrane receptors, are destroyed after pronase treatment (18). The uptake of Aco-HSA liposomes by liver parenchymal cells, which constitute by far the largest cell population in the liver, was only small. On a per-cell basis the uptake by hepatocytes was only 5% of that by the endothelial cells. Preinjection with polyinosinic acid substantially reduced the uptake of Aco-HSA liposomes by endothelial and Kupffer cells to 21% and 34% of the control values, respectively (P < 0.01). Also the small uptake by parenchymal cells was reduced to 43% of the control value. Because of the small amount of control liposomes taken up by the liver 30 min after injection (only ≈5% of injected dose), we isolated liver endothelial and Kupffer cells from rats injected with control liposomes at 3 hr after injection. At that time 24.8 ± 6.5% of the injected dose was recovered in the liver, primarily present in the nonparenchymal cells. Within this population only Kupffer cells contributed significantly to the uptake of control liposomes (2.9 ± 0.5 nmol of total lipid per 1⋅106 cells), whereas endothelial cells were virtually devoid of liposomal label (0.06 nmol of total lipid per 106 cells). Because endothelial cells are not normally phagocytic and therefore are not likely to internalize large particles, we determined the effect of liposomal size on the uptake of Aco-HSA liposomes by these cells (Table 1). Increase of liposome size resulted in a decrease in uptake of the anionized liposomes by endothelial cells. Uptake by endothelial cells of liposomes extruded through filters with a pore size of 400 nm was reduced by about 65% as compared with 50-nm extruded liposomes. Kupffer cell uptake of liposomes gradually increased with size. Liposomes extruded through filters with a pore size of 400 nm are taken up more than 5-fold more efficiently by these cells than liposomes extruded through 50-nm pore size filters.
Figure 1.
Tissue distribution of control liposomes (empty bars) and Aco-HSA liposomes (filled bars) 30 min after intravenous injection. [3H]cholesteryl oleyl ether-labeled liposomes (1 μmol of total lipid/100 g body weight) were injected into anesthetized rats. Tissue distribution was determined as described in Materials and Methods. Data are presented as means ± SD of 3–5 rats.
Figure 2.
The effect of polyinosinic acid on tissue distribution of Aco-HSA liposomes 30 min after intravenous injection. [3H]cholesteryl oleyl ether-labeled liposomes (1 μmol of total lipid/100 g body weight) were injected into anesthetized rats, which were untreated (filled bars) or treated with 5 mg polyinosinic acid 1 min before injection with liposomes (hatched bars). Tissue distribution was determined as described in Materials and Methods. Data are presented as means ± SD of 3–4 rats.
Figure 3.
Intrahepatic distribution of Aco-HSA liposomes 30 min after intravenous injection. [3H]cholesteryl oleyl ether-labeled liposomes (1 μmol of total lipid/100 g body weight) were injected into anesthetized rats, which were untreated (filled bars) or treated with 5 mg polyinosinic acid 1 min before injection with liposomes (hatched bars). Intrahepatic distribution was determined as described in Materials and Methods. Data are presented as means ± SD of 1–4 rats. EC, endothelial cells; KC, Kupffer cells; PC, parenchymal cells.
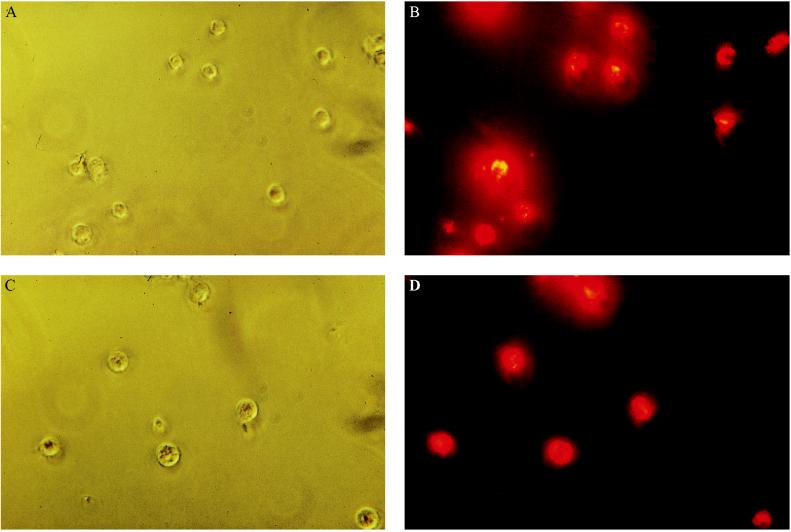
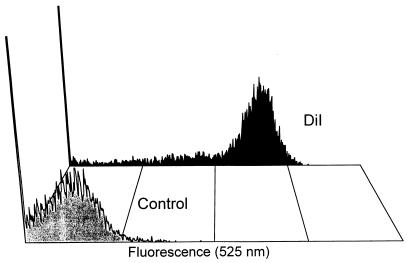
Fluorescence microscopy of liver endothelial and Kupffer cells, which were isolated 30 min after injecting a rat with fluorescently labeled Aco-HSA(DiI) liposomes, demonstrated that in both cell populations in the liver more than 80% of the cells contributed to the uptake of these liposomes (Fig. 4). These results were confirmed by flow cytometry, as is shown for endothelial cells in Fig. 5. This figure clearly indicates that the vast majority of the cells produced a fluorescence signal that is more than 1,000-fold increased over the autofluorescence signal of the control cells.
Figure 4.
Visualization of the uptake of Aco-HSA liposomes by liver endothelial and Kupffer cells 30 min after intravenous injection. DiI-labeled liposomes (1 μmol of total lipid/100 g body weight) were injected into anesthetized rats. Endothelial (A and B) cells and Kupffer (C and D) cells were isolated and the liposomes were visualized as described in Materials and Methods. A and C phase contrast. B and D fluorescence of the same field. (Original magnification ×400.)
Figure 5.
Representative flow cytometer plots of liver endothelial cells 30 min after intravenous injection of buffer [10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Hepes)/135 mM NaCl, pH 6.7] (control) or DiI-labeled Aco-HSA liposomes. DiI-labeled liposomes (1 μmol of total lipid/100 g body weight) or buffer were injected into anesthetized rats. Endothelial cells were isolated and subjected to flow cytometry as described in Materials and Methods. Fluorescence at 525 nm is depicted on a log scale.
DISCUSSION
In various animal species and humans, liposomes are generally cleared rapidly by the cells of the mononuclear phagocyte system, especially macrophages in the spleen and Kupffer cells in the liver (19, 20). When liposomes are small enough to be able to pass the fenestrae in the endothelial cell lining, they also will have access to the hepatocytes and be taken up by these cells (19). To the best of our knowledge no reports in the literature have described significant uptake of liposomes of any composition by endothelial cells in vivo. We and others have repeatedly found and reported the actual lack of involvement of liver endothelial cells in clearance of liposomes from the blood (21).
Coupling of Aco-HSA to liposomes leads to a 17-fold increase in liver uptake, as compared with control liposomes, whereas spleen uptake is not affected. Within the liver, Aco-HSA liposomes are mainly taken up by the sinusoidal endothelial and the Kupffer cells. Expressed per number of cells the uptake of Aco-HSA liposomes by endothelial cells is of the same order of magnitude as that by Kupffer cells. Because rat liver contains three times as many endothelial cells as Kupffer cells however, our data show that the endothelial cell population accounts for a considerably larger fraction of the hepatic uptake of Aco-HSA liposomes than the Kupffer cells. From the uptake per cell and published data on liver cell numbers (194⋅106 nonparenchymal and 450⋅106 parenchymal cells per 100 g body weight) (22), it can be calculated that liver endothelial cells contribute to an extent of 64.3% to the total liver uptake of Aco-HSA liposomes and Kupffer cells 25.3%. The remaining 10.4% is accounted for by binding to or uptake in parenchymal cells. Confirming earlier observations from our laboratory, control liposomes are virtually taken up only by the Kupffer cells (80%) and, to a small extent, by hepatocytes, whereas the sinusoidal endothelial cells do not significantly contribute.
The high endothelial cell uptake of Aco-HSA liposomes probably is related to the abundant presence of scavenger receptors on this cell type, which have been demonstrated to be responsible for the uptake of a large variety of polyanionic macromolecules, including negatively charged albumins (11). Involvement of macrophage scavenger receptors also has been suggested in the uptake of liposomes that were negatively charged by conjugation with polyacrylic acid (23). To verify the involvement of these scavenger receptors in the uptake of Aco-HSA liposomes, rats were preinjected with polyinosinic acid, a well known inhibitor of scavenger receptor mediated uptake. The efficient inhibition of the uptake of Aco-HSA liposomes by endothelial and Kupffer cells caused by polyinosinic acid indicates that scavenger receptors are involved in the uptake of these liposomes. Different types of scavenger receptors have been identified (24) up to now, several of which on the different liver cell types, including endothelial cells (10, 25). We have no indication that the Aco-HSA liposomes also interact to a significant extent with extrahepatic vascular endothelium: liver and spleen account for >90% of the liposomes cleared from the blood. It remains to be clarified which scavenger receptor type on the endothelial and Kupffer cells is responsible for Aco-HSA liposome uptake, but it is likely to be one that is not present on extrahepatic vascular endothelial cells.
The ability of Kupffer cells to take up large particles is well established. In none of our previous studies we ever observed, however, significant participation of the liver endothelial cells in liposome elimination from the blood. Other studies have shown the ability of endothelial cells to endocytose large particles, such as polystyrene particles coated with transferrin (26). Increasing the size of Aco-HSA liposomes led to a decrease in uptake by liver endothelial cells whereas Kupffer cell uptake was increased. Consequently, the smaller (<100 nm) Aco-HSA liposomes are most efficiently taken up by the hepatic endothelial cells.
Polyanionic ligands, including polyanionized albumins, generally are considered appropriate ligands for scavenger receptors (11, 24). This, together with the inhibitory action of polyinosinic acid on liposome uptake by the endothelial cells, leads us to believe that our Aco-HSA liposomes interact with the hepatic endothelial cells through a scavenger receptor. It is clear, however, that the receptor also displays conformational requirements of the negative charge cluster. We observed that intravenously administered liposomes containing up to 100% of the negatively charged phospholipid phosphatidylserine do not interact at all with the endothelial cells (27). Our study also tells us that, in addition to the requirement of a polyanionic structure, the receptor has requirements with respect to ligand size; with increasing liposome size the binding to the endothelial cells rapidly diminished. This may be related to the positioning of the receptor in coated pits, which would set limits to the size of particles that can be bound (28).
In conclusion, we have shown that small Aco-HSA liposomes can be targeted with high efficiency to liver endothelial cells. This targeting is not fully specific, because Kupffer cells and to some extent hepatocytes also participate in the clearance process. We have shown massive delivery of a very substantial proportion of an i.v.-administered dose of liposomes to a well defined population of cells other than macrophages (or hepatocytes). This opens up attractive possibilities to modulating the function of this cell type with liposome-encapsulated agents, like antisense oligonucleotides, or plasmid DNA. The sinusoidal endothelial cells produce a variety of cytokines and lipid mediators crucial in the homeostasis of liver functions but also are involved in various liver-associated disorders (29, 30).
Because it has been reported that both sinusoidal endothelial and Kupffer cells are susceptible to infection with HIV type 1 (31, 32), Aco-HSA liposomes also may act as a highly specific drug carrier system of anti-HIV type 1 drugs to these cells (30, 33).
Table 1.
Effect of the size of Aco-HSA liposomes on the uptake by liver endothelial cells and Kupffer cells 30 min after intravenous injection
| Pore size, nm* | Uptake, μmol total lipid/1⋅106 cells
|
|
|---|---|---|
| Endothelial cells | Kupffer cells | |
| 50 | 2.8 ± 0.5† | 3.3 ± 0.5† |
| 100 | 1.9 ± 0.1 | 7.4 ± 0.0 |
| 200 | 1.0 ± 0.1 | 15.7 ± 1.9 |
| 400 | 1.0 ± 0.1 | 17.6 ± 2.2 |
[3H]cholesteryloleyl-ether labeled liposomes (1 μmol of total lipid/100 g body weight), extruded through filters with the indicated pore size, were injected into anesthetized rats. Intrahepatic distribution was determined as described in Materials and Methods. Data are presented as means ± half the difference between the two values of two rats or
as means ± SD of three rats.
Actual average diameters after extrusion through filters with the indicated pore size were 92.1 nm (50 nm), 153.5 nm (100 nm), 263.2 nm (200 nm), and 590.7 nm (400 nm).
Acknowledgments
We thank Bert Dontje for excellent technical assistance with animal handling. We thank G. Mesander (Shared Flow Cytometry Facilities, Faculty of Medical Sciences University of Groningen, Groningen, The Netherlands) for FACS operation. This research was financially supported by the Netherlands Organization for Scientific Research, Grant No. 902-23-187.
ABBREVIATIONS
- HSA
human serum albumin
- Aco-HSA
polyaconitylated human serum albumin
- DiI
1,1′dioctadecyl-3,3,3′,3′-tetramethyl indocarbocyanine perchlorate
References
- 1.Thierry A R, Rahman A, Dritschilo A. In: Gene Regulation: Biology of Antisense RNA and DNA. Erickson R P, Izant J G, editors; Erickson R P, Izant J G, editors. New York: Raven; 1992. pp. 147–161. [Google Scholar]
- 2.Lasic D D, Templeton N S. Adv Drug Delivery Rev. 1996;20:221–266. [Google Scholar]
- 3.Mahato R I, Kawabata K, Takakura Y, Hashida M. J Drug Targeting. 1995;3:149–157. doi: 10.3109/10611869509059214. [DOI] [PubMed] [Google Scholar]
- 4.Litzinger D C, Brown J M, Wala I, Kaufman S A, Van G Y, Farrell C L, Collins D. Biochim Biophys Acta. 1996;1281:139–149. doi: 10.1016/0005-2736(95)00268-5. [DOI] [PubMed] [Google Scholar]
- 5.Allen T M. Trends Pharmacol Sci. 1994;15:215–220. doi: 10.1016/0165-6147(94)90314-x. [DOI] [PubMed] [Google Scholar]
- 6.Mori A, Kennel S J, Van Borssum Waalkes M, Scherphof G L, Huang L. Cancer Chemother Pharmacol. 1995;35:447–456. doi: 10.1007/BF00686828. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Molema G, King S, Watkins L, Edgington T S, Thorpe P E. Science. 1997;275:547–550. doi: 10.1126/science.275.5299.547. [DOI] [PubMed] [Google Scholar]
- 8.Jansen R W, Schols D, Pauwels R, De Clercq E, Meijer D K F. Mol Pharmacol. 1993;44:1003–1007. [PubMed] [Google Scholar]
- 9.Kamps J A A M, Swart P J, Morselt H W M, Pauwels R, De Béthune M P, De Clercq E, Meijer D K F, Scherphof G L. Biochim Biophys Acta. 1996;1278:183–190. doi: 10.1016/0005-2736(95)00218-9. [DOI] [PubMed] [Google Scholar]
- 10.Van Berkel Th J C, De Rijke Y B, Kruijt J K. J Biol Chem. 1991;266:2282–2289. [PubMed] [Google Scholar]
- 11.Jansen R W, Molema G, Harms G, Kruijt J K, Van Berkel Th J C, Hardonk M J, Meijer D K F. Biochem Biophys Res Commun. 1991;180:23–32. doi: 10.1016/s0006-291x(05)81249-5. [DOI] [PubMed] [Google Scholar]
- 12.Lowry O H, Rosenbrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.Böttcher C J F, Van Gent C M, Pries C. Anal Chim Acta. 1961;24:203–204. [Google Scholar]
- 14.Bijsterbosch M K, Ziere G J, Van Berkel Th J C. Mol Pharmacol. 1989;36:484–489. [PubMed] [Google Scholar]
- 15.Daemen T, Veninga A, Roerdink F H, Scherphof G L. Cancer Res. 1986;46:4330–4335. [PubMed] [Google Scholar]
- 16.Casteleijn E, Van Rooij H C J, Van Berkel Th J C, Koster J F. FEBS Lett. 1986;201:193–194. doi: 10.1016/0014-5793(86)80607-x. [DOI] [PubMed] [Google Scholar]
- 17.Scherphof G L, Velinova M, Kamps J A A M, Donga J, Van der Want H, Kuipers F, Havekes L, Daemen T. Adv Drug Delivery Rev. 1997;24:179–191. [Google Scholar]
- 18.Nagelkerke J F, Barto K P, Van Berkel Th J C. J Biol Chem. 1983;258:12221–12227. [PubMed] [Google Scholar]
- 19.Roerdink F, Dijkstra J, Hartman G, Bolscher B, Scherphof G L. Biochim Biophys Acta. 1981;677:79–89. doi: 10.1016/0304-4165(81)90148-3. [DOI] [PubMed] [Google Scholar]
- 20.Scherphof G L, Morselt H, Allen T M. J Liposome Res. 1994;4:213–228. [Google Scholar]
- 21.Spanjer H H, Van Galen M, Roerdink F H, Regts J, Scherphof G L. Biochim Biophys Acta. 1986;863:224–230. doi: 10.1016/0005-2736(86)90262-2. [DOI] [PubMed] [Google Scholar]
- 22.Kooistra T, Duursma A M, Bouwma J M W, Gruber M. Biochim Biophys Acta. 1979;587:282–298. doi: 10.1016/0304-4165(79)90361-1. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara M, Baldeschwieler J D, Grubbs R H. Biochim Biophys Acta. 1996;1278:59–67. doi: 10.1016/0005-2736(95)00183-2. [DOI] [PubMed] [Google Scholar]
- 24.Krieger M, Herz J. Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 25.Kamps J A A M, Kruijt J K, Kuiper J, Van Berkel Th J C. Arterioscler Thromb. 1992;12:1079–1087. doi: 10.1161/01.atv.12.9.1079. [DOI] [PubMed] [Google Scholar]
- 26.Soda R, Tavassoli M. Blood. 1984;63:270–276. [PubMed] [Google Scholar]
- 27.Kamps J A A M, Morselt H W M, Scherphof G L. Prog Drug Delivery Syst. 1996;5:89–92. [Google Scholar]
- 28.Esbach S, Pieters M N, Van der Boom J, Schouten D, Van der Heyde M N, Roholl P J, Brouwer A, Van Berkel Th J C, Knook D L. Hepatology. 1993;18:537–545. [PubMed] [Google Scholar]
- 29.Rieder H, Meyer zum Büschenfelde K H, Ramadori G. J Hepatol. 1992;15:237–250. doi: 10.1016/0168-8278(92)90042-n. [DOI] [PubMed] [Google Scholar]
- 30.Meijer D K F, Molema G. Semin Liver Dis. 1995;15:202–256. doi: 10.1055/s-2007-1007278. [DOI] [PubMed] [Google Scholar]
- 31.Housset C, Lamas E, Courgnaud V, Boucher O, Girard P M, Marche C, Brechot C. J Hepatol. 1993;19:252–258. doi: 10.1016/s0168-8278(05)80579-3. [DOI] [PubMed] [Google Scholar]
- 32.Lafon M E, Steffan A M, Royer C, Jaeck D, Beretz A, Kirn A, Gendrault J L. AIDS. 1994;8:747–752. doi: 10.1097/00002030-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Meijer D K F, Jansen R W, Molema G. Antiviral Res. 1992;18:215–248. doi: 10.1016/0166-3542(92)90058-d. [DOI] [PubMed] [Google Scholar]